TURNING BACK THE CLOCK
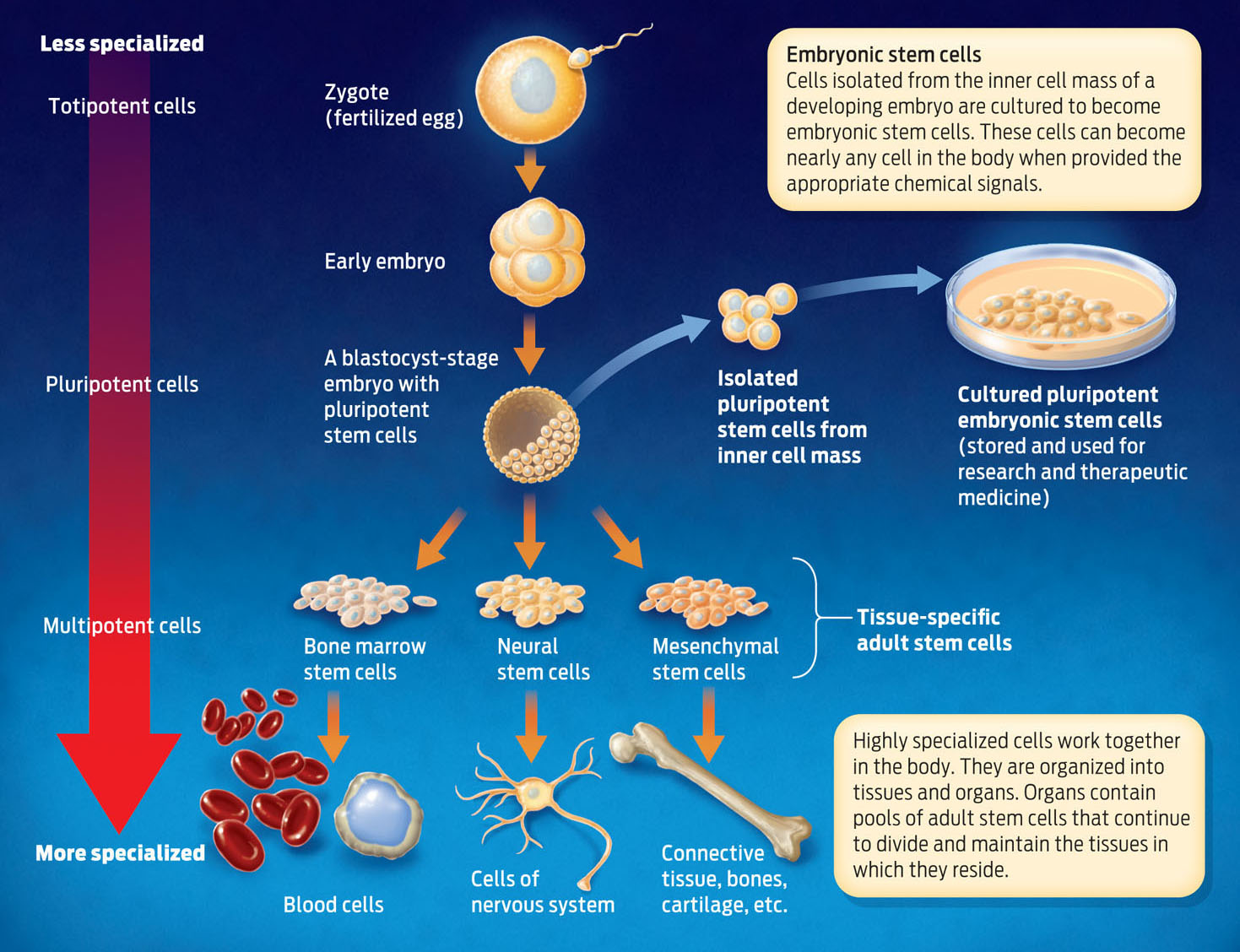
MULTIPOTENT Describes a cell with the ability to differentiate into a limited number of cell types in the body.
EMBRYONIC STEM CELLS Stem cells that make up an early embryo and which can differentiate into nearly every cell type in the body.
Because of their remarkable capacity to produce new cells that can differentiate and specialize, stem cells show great promise in regenerative medicine. But it turns out that not all stem cells are created equal. Adult stem cells–those found in mature tissues–typically can differentiate only into one or a few cell types. Most bone marrow stem cells, for example, in normal circumstances differentiate only into blood cells–but not into neurons or bladder cells. Such cells with restricted ability to differentiate are described as multipotent.
BLASTOCYST The stage of embryonic development in which the embryo is a hollow ball of cells. Researchers can derive embryonic stem cell lines during the blastocyst stage.
Other stem cells–those active during embryonic development, for example–can differentiate into many more cell types. Such embryonic stem cells are found in early embryos at what’s known as the blastocyst stage, when the embryo is mostly a hollow ball of cells. Unlike adult stem cells, which differentiate only into certain cell types, embryonic stem cells can give rise to nearly any cell type in the body. For this reason, they are referred to as pluripotent. At even earlier stages of embryonic development, embryonic cells can differentiate into every cell type, and are therefore described as totipotent (INFOGRAPHIC 13.6) .
PLURIPOTENT Describes a cell with the ability to differentiate into nearly any cell type in the body.
TOTIPOTENT Describes a cell with the ability to differentiate into any cell type in the body.
Embryonic stem cells (ESC) come from embryos and have different characteristics than adult cells. ESCs can develop into almost any cell type, and therefore may have a greater therapeutic potential compared to adult stem cells.
293
Some scientists argue that embryonic stem cells hold greater potential in treating disease because they are not as specialized as adult stem cells and can therefore differentiate into many more cell types. With embryonic stem cells, scientists can in principle make any cell type they need.
The challenge has always been how to obtain them. One source of embryonic stem cells is discarded human embryos from fertility clinics. Scientists can extract cells from an embryo and place them in media that stimulates them to divide. These cells are then stored, used in research, and could potentially be used in treatment. But that became much harder to do when, in 2001, President George W. Bush put in place severe restrictions on the use of federal funds to support research using stem cells derived from embryos. By this mandate, only a small number of existing cell lines were eligible for federal funding, and funding for making new stem cell lines was prohibited. In 2009, by executive order, President Barack Obama removed some of these restrictions, but funding remains controversial with many members of Congress.
Another way to obtain embryonic stem cells is by a technique called cloning. In this method, scientists replace the nucleus of a haploid unfertilized human egg with the diploid nucleus taken from another cell (a skin cell, for example). The chemical “soup” inside the egg turns on specific genes in the donated nucleus, resetting it to an embryonic state. This technique, known as somatic cell nuclear transfer (SCNT), produces a new embryo with the same genes as the donor cell. This is how Dolly, the first cloned sheep, was created in 1996 (INFOGRAPHIC 13.7) .
Somatic cell nuclear transfer, or cloning, involves replacing the nucleus of an egg with a nucleus from a specialized cell, creating an embryo that is genetically identical to the donor cell.
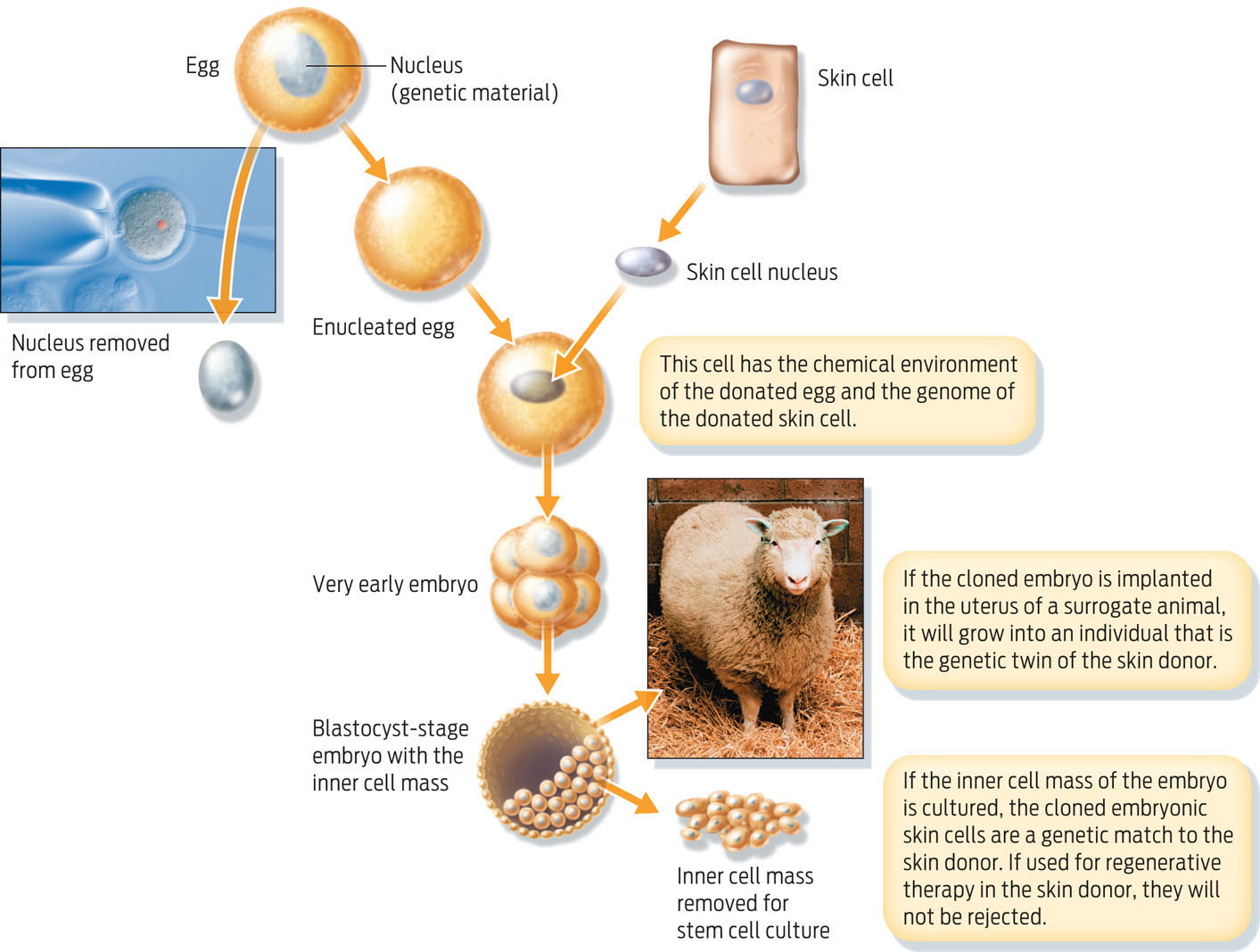
If scientists were to implant a cloned embryo in a woman’s womb, it would develop into a fetus that has the same nuclear DNA as the person who donated the cells from which the diploid nucleus was taken. While such reproductive cloning is prohibited in the United States, scientists funded by nongovernmental sources are allowed to produce cloned embryos for research–so-called “therapeutic cloning.” Embryonic stem cells made by SCNT can be extracted from the new embryo and grown in a petri dish to form a population of stem cells that are essentially genetically identical to the donor. Consequently, any differentiated cells derived from these stem cells could be transplanted back into the donor without fear of an immune response against the transplanted cells.
The main ethical difficulty with both sources of embryonic stem cells–frozen embryos from fertility clinics and cloned embryos generated in the lab–is that human embryos are destroyed in the process. To date, most embryonic stem cell lines in the United States have been derived from embryos stored at fertility clinics and subsequently donated to research. If not donated to science, these extra embryos would be destroyed after a period of time anyway. Nevertheless, many people find the idea of deliberately interfering with human embryos troubling.
294
INDUCED PLURIPOTENT STEM CELL A pluripotent stem cell that was generated by manipulation of a differentiated somatic cell.
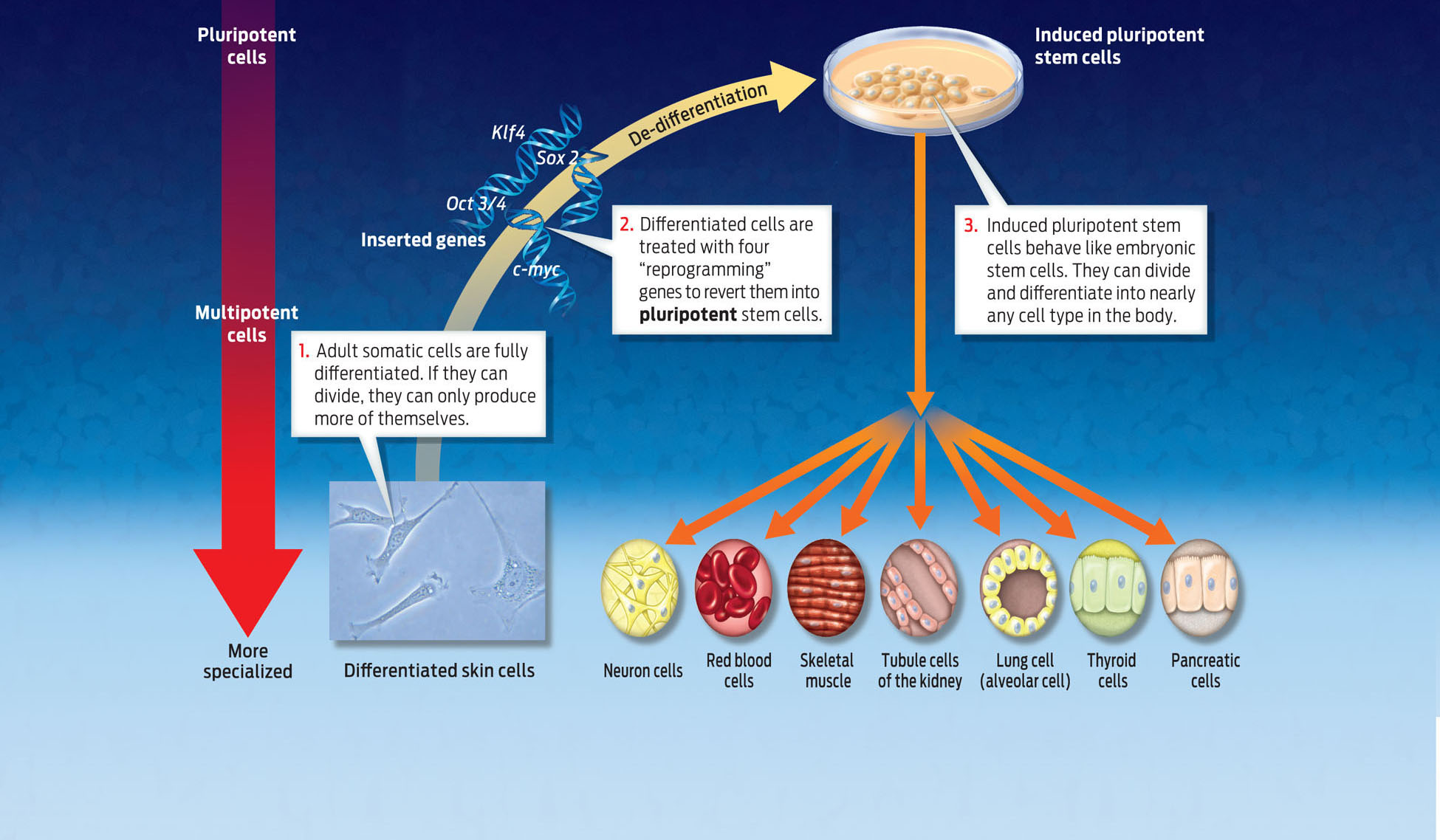
Keenly aware of the ethical challenges, the scientific community has been searching for other methods to generate embryonic stem cells. In 2007, scientists accomplished a feat that could make the ethical controversy obsolete. They discovered a way to produce embryonic-like stem cells without touching an embryo. They did this by reprogramming mature cells, converting them into cells that to all appearances behave exactly like embryonic stem cells, capable of dividing and differentiating into any cell type.
In 2007, James Thompson at the University of Wisconsin and Shinya Yamanaka of Kyoto University in Japan independently showed that they could turn a mature human cell–one that has already differentiated–into an “embryonic” stem cell by inserting four human genes into its genome. They called the new cells induced pluripotent stem cells to reflect the fact that mature cells had been genetically manipulated to become embryonic-like stem cells. Yamanaka, for example, took an adult skin cell and inserted four human genes into it–genes that are normally switched off in skin cells. The added genes produced proteins that were able to turn off the expression of genes associated with the differentiated state and turn on genes associated with pluripotency. By changing gene expression in this way, the researchers were able to “de-differentiate” the skin cell–to turn back the clock, in a sense–and return the cell to its embryonic state. It was a major technological breakthrough. This promising technique may offer a way to produce transplantable cells that are genetically matched to a patient without depending on embryos. For his pioneering work, Yamanaka won a Nobel prize in 2012 (INFOGRAPHIC 13.8) .
295
Atala’s group has also shown that it is possible to isolate embryonic-like stem cells from amniotic fluid, another potentially valuable way of obtaining them.
One method of creating embryonic stem cells is to induce adult stem cells to de-differentiate. Scientists have done just that by inserting either specific genes or proteins into adult cells. The expression of these genes causes the differentiated cells to act like pluripotent stem cells. This technology offers the potential to create immune matched stem cells for therapy, just like cloning, but it does not involve destroying an embryo.
Research using both embryonic and adult stem cells in regenerative medicine is forging ahead. In 2012, scientists at Advanced Cell Technology, a biotechnology company in Massachusetts, reported that they had partially restored vision to two legally blind patients suffering from an eye disease called macular degeneration. The stem cells were injected directly into the patients’ eyes, where they differentiated into new retina cells. And researchers at Emory University and the University of Michigan are conducting a clinical trial of embryonic stem cells in patients with ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease), with some initial positive results: a few patients have regained the ability to move some previously paralyzed muscles.
296
Even the dumbest stem cell is smarter than the smartest neuroscientist.
–EVAN SNYDER
As for how the stem cells are working on a chemical level to heal damaged tissue, researchers are the first to admit they don’t know. “Even the dumbest stem cell is smarter than the smartest neuroscientist,” says Evan Snyder, program director at the Burnham Institute for Medical Research in La Jolla, California. “The cells are making stuff that we might not be able to identify for centuries.” But that hasn’t stopped researchers from attempting to use stem cells for therapeutic purposes.