GOT MILK?
More than 20 years ago, when Harry Meade was working as a research scientist at a company called Biogen, it occurred to him that producing drugs in a mammal’s milk might be more efficient than obtaining proteins from blood. Meade, who grew up on a dairy farm outside Pittsburgh, Pennsylvania, reasoned that the mammary gland is a natural protein factory. To nourish their young, all mammals produce proteins and secrete them into their milk.
At the time of his eureka moment, Meade had been experimenting with putting human genes into mice, which would then produce the human proteins in their blood. The method worked, but in order to obtain the proteins, the mice had to be killed to harvest their blood. Meade realized that if he could get mice or other animals to produce the proteins in their milk, then they wouldn’t have to be bled-they could be milked instead.
169
“I was reminded of the fable about the goose that laid a golden egg each day,” wrote Meade in an article for The Sciences in 1997. “Killing the goose left the owner with nothing, while caring for it patiently would have yielded a lifetime of steady benefits.” Using a larger mammal, like a goat or cow, would be even better than using a mouse, because the yield would be much greater.
TRANSGENIC Refers to an organism that carries one or more genes from a different species.
To develop this idea, in 1994, Meade co-founded GTC Biotherapeutics (renamed rEVO Biologics in 2012). The company decided to use goats because they produce ample milk but have faster gestation times and are cheaper to maintain than cows.
REGULATORY SEQUENCE The part of a gene that determines the timing, amount, and location of protein production.
Animals that have been genetically modified to contain genes from other species are called transgenic organisms (“trans” means “across”–in this case, across species, from one to another). The basic idea for making a transgenic goat is simple: isolate the gene of interest from a human chromosome and then insert it into the genome of a goat embryo; the goat grows up containing the human gene in its cells. But in order to make sure the human gene is expressed when the goat produces milk, Meade and his colleagues had to create a hybrid gene that was part human and part goat.
CODING SEQUENCE The part of a gene that specifies the amino acid sequence of a protein. Coding sequences determine the identity, shape, and function of proteins.
Genes are organized into two parts. Regulatory sequences determine when and how much protein a gene makes. Coding sequences determine the amino acid sequence of the encoded protein, which determines its shape and function.
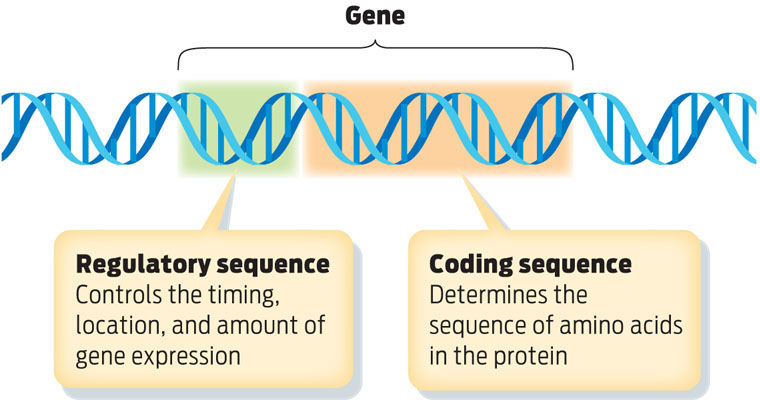
Meade’s technique made use of the fact that every gene has two parts: a regulatory sequence and a coding sequence. Regulatory sequences are like on/off switches for genes; by providing a site where regulatory molecules can bind, they determine when, where, and how much protein a gene makes. Coding sequences determine the identity of a protein: they specify the order, or sequence, of amino acids. Together, the regulatory sequence and the coding sequence ensure that every gene is expressed only at the right time and in the right cells (INFOGRAPHIC 8.5) .
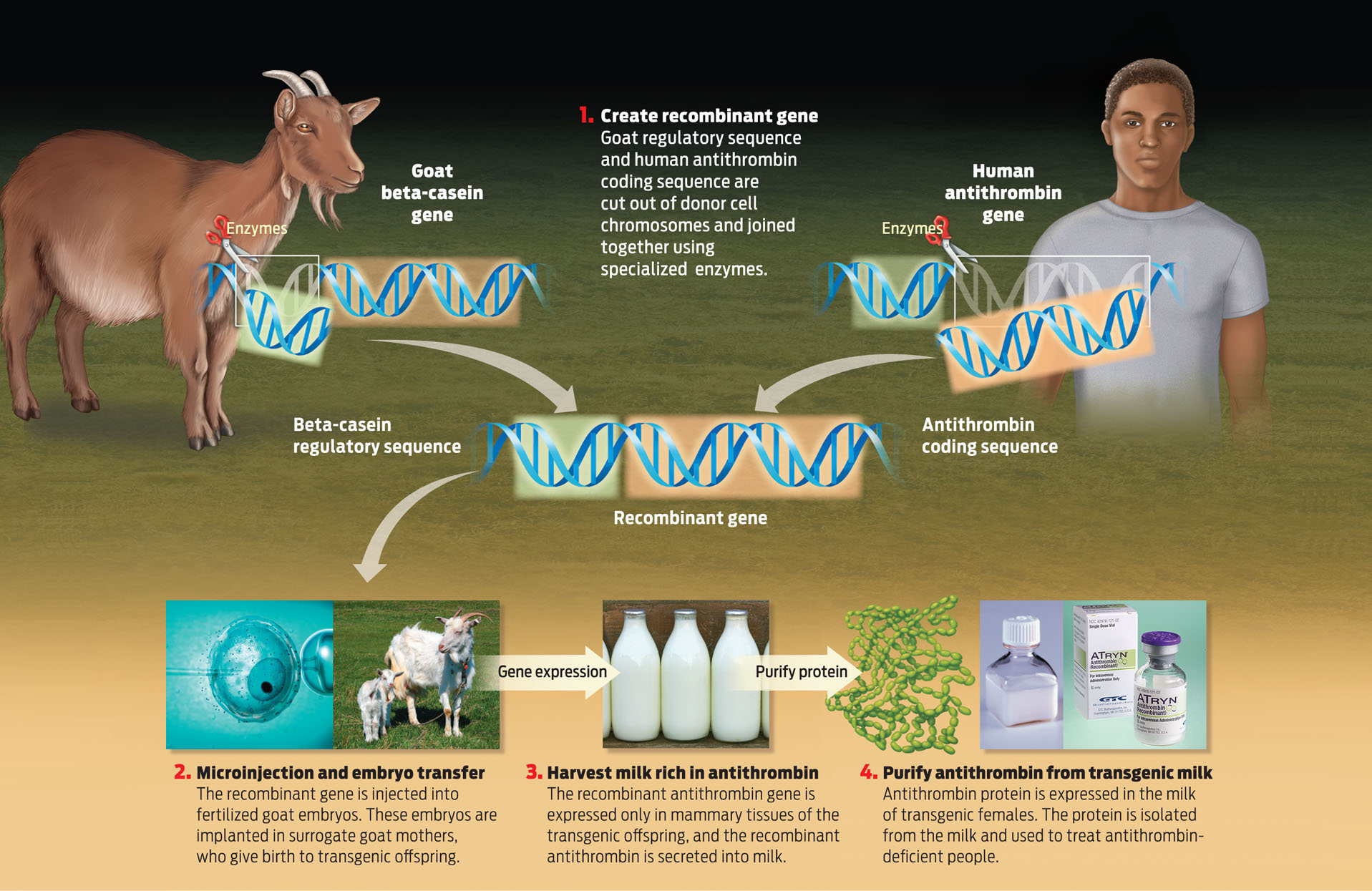
Meade realized that if he could attach the coding sequence of the human antithrombin gene to the regulatory sequence of a goat gene that is expressed in the animal’s mammary cells, he could get the goat’s mammary cells to make the human protein. In other words, with this gene construct he could dupe the goat’s mammary glands into making the human antithrombin protein and secreting it as part of the goat’s milk when the animal lactated.
GENETIC ENGINEERING The process of assembling new genes with novel combinations of regulatory and coding sequences.
First, the team had to find the right goat gene to work with. That part was easy. About 80% of milk proteins are caseins (from the Latin caseus, meaning “cheese”). This family of proteins is produced in the mammary gland and nowhere else in the animal, making these proteins an obvious choice. The team chose one particularly well-characterized casein gene, beta-casein, which had already been studied in mice.
RECOMBINANT GENE A genetically engineered gene.
Next, using genetic engineering techniques they spliced together the regulatory sequence of this milk gene and the coding sequence of the human antithrombin gene. A genetically engineered hybrid gene like this one is often referred to as a recombinant gene. By using the regulatory sequence of the milk gene in their recombinant gene, researchers could ensure that antithrombin is expressed only in the mammary cells, and not in any other tissues. That’s important because proteins expressed where they shouldn’t be can cause problems.
170
If all cells have the same DNA (and they do), why is beta-casein protein–and therefore antithrombin–produced only in mammary cells? It turns out that different cell types contain different suites of proteins; in fact, it is this unique set of proteins that gives each cell type its identity (see Chapter 13). Only mammary cells respond to the signal to produce milk, and only mammary cells have the necessary proteins to bind to the regulatory sequence of the beta-casein gene, turning it on and causing it to be expressed. In other words, the beta-casein regulatory sequence acts as a switch. “So you put the gene in and you put a switch in, and the switch only gets turned on in the mammary gland,” explains Simon Lowry, Vice President and Head of Medical Affairs at rEVO.

With the recombinant gene in hand, researchers then began the delicate process of putting this hybrid gene into a goat. Here’s how it works: While looking through a microscope, scientists use a needle to inject the recombinant gene into a fertilized single-cell goat embryo. This transgenic embryo is then implanted into a surrogate mother. As the embryo grows and the cells divide, the inserted gene is replicated and passed on to every cell in the developing goat. If this goat is female, it can be used to obtain medicine-rich milk. The goat can also be bred with other goats to create a herd of “pharm” animals.
But the process is harder than it sounds, and took a lot of trial and error. Early attempts had less than a 5% success rate. For that reason, explains Meade, producing a single transgenic founder animal requires implanting 100 to 200 microinjected embryos. The good news is that once a founder goat is produced, it can be bred to create an endless supply of new transgenic goats. From start to finish, it took about 15 years to create a herd of transgenic goats. By 2006, the researchers were in business (INFOGRAPHIC 8.6) .
GENETICALLY MODIFIED ORGANISM (GMO) An organism that has been genetically altered by humans.
171
GENETHERAPY A treatment that aims to cure human disease by replacing defective genes with functional ones.
Animals aren’t the only organisms that have been genetically modified by humans. Much of the corn we eat today is transgenic-it contains genes from a soil bacterium. There are strains of transgenic soybeans, transgenic tomatoes, and transgenic insects. Transgenic organisms are also called genetically modified organisms (GMOs). Transgenic crops such as corn and soybeans usually contain genes for natural pesticides, which help the plants fight pests and reduce the amount of pesticide a farmer must use. Others varieties contain pesticide-resistance genes, allowing farmers to spray pesticides on fields to kill weeds without at the same time killing crops.
Such gene-swapping technology also has an important application in medicine: in gene therapy scientists attempt to replace a defective human gene with a healthy one, an approach that is already treating, and in some cases curing, debilitating diseases such as severe combined immunodeficiency syndrome (SCID)–a disorder in which babies are born with deficient immune systems. Researchers hope that gene therapy may one day help treat several disorders caused by defective genes, including cystic fibrosis, Huntington disease, and hemophilia.
172
Despite the many actual and potential benefits of genetic engineering, mixing and matching genes inspires debate among scientists, environmentalists, and the general public. Many people object to human meddling with the biology of organisms that have evolved naturally. Others worry what might happen to a natural population of organisms-such as corn plants-if their genetically modified cousins (which contain, for example, pesticide-resistance genes) were to escape into the environment and cross with the unmodified population; the consequences are unpredictable.
The fear that meat, cheese, or milk from the transgenic goats might make its way into the human food supply was an explicit concern voiced by environmentalists when ATryn was being evaluated by the FDA. The FDA representative who reviewed the case testified that rEVO has mechanisms in place to make sure that doesn’t happen: a double-fenced facility, video cameras on the grounds, and homing devices implanted under the goats’ skin to help locate a goat in the unlikely event that one were to escape. But not everyone is convinced. “Humans are fallible and accidents do happen,” said Gregory Jaffe, director of the Biotechnology Project of the Center for Science in the Public Interest in an interview with Scientific American in 2009. “Even if they’re not intended to end up in the food supply, many things do end up in the food supply.”
Although the idea of genetically engineering organisms is disquieting to some, others point out that humans have been tampering with the natural evolution of plants and animals for centuries by selectively breeding them for desirable traits. Moreover, from an animal-rights point of view, transgenic goats are no worse off than goats farmed for their milk and meat. (In fact, Meade’s goats are treated better–they have generous room to run around, are given toys to play with, stairs to climb, even backscratchers to keep them comfortable.)
Being able to genetically modify organisms for human purposes raises legitimate questions about how to conduct genetic engineering safely and humanely. For example, many people who find nothing ethically troubling about using gene therapy to treat human diseases nonetheless find the prospect of using it to change personality or sexual orientation more problematic. Scientists can now use genetic engineering technology to clone entire animals–growing them from a single cell (Dolly the sheep was the first of these, in 1996). Will humans be next? Many people find that possibility disturbing. With the goats, however, genetic engineering is being used to save human lives–a much less controversial use of the technology.
Karen James, a New Mexico lawyer whose 17-year-old daughter died of a massive blood clot in her brain due to inherited antithrombin deficiency, spoke at the FDA hearing in 2009: “As a wife, as a mother, as someone who would like to be a grandmother someday, I’m here to tell you that antithrombin deficiency is the stuff of nightmares,” she said. As long as the animals are not hurt by the practice, James said she has no problems with this method being used to obtain medicine. “If it helps other families avoid tragedies like ours, I think it’s a wonderful idea.”
 As a wife, as a mother, as someone who would like to be a grandmother someday, I’m here to tell you that antithrombin deficiency is the stuff of nightmares.
As a wife, as a mother, as someone who would like to be a grandmother someday, I’m here to tell you that antithrombin deficiency is the stuff of nightmares.
— KAREN JAMES
173