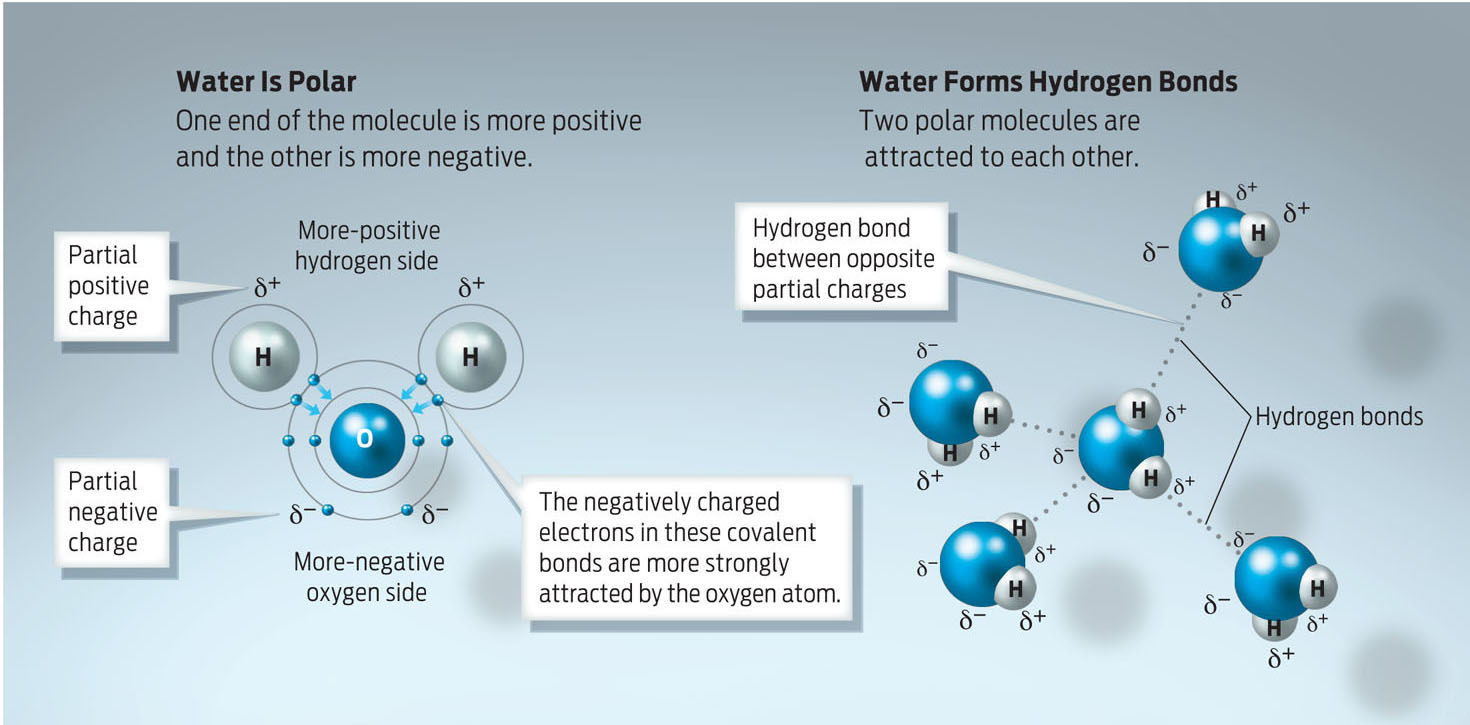
INFOGRAPHIC 2.6 WATER IS POLAR AND FORMS HYDROGEN BONDS
Water is a polar molecule because electrons are not shared equally between the oxygen and the hydrogen atoms in each of its polar covalent bonds. Electrons are pulled closer to the oxygen atom than to the hydrogen atoms, creating a slightly negative oxygen atom and a slightly positive hydrogen atom. When many water molecules are near one another, the partially positive hydrogen atoms of some molecules are attracted to the partially negative oxygen atoms of neighboring water molecules. These attractions are hydrogen bonds, weak electrical attractions.
Water is a polar molecule because electrons are not shared equally between the oxygen and the hydrogen atoms in each of its polar covalent bonds. Electrons are pulled closer to the oxygen atom than to the hydrogen atoms, creating a slightly negative oxygen atom and a slightly positive hydrogen atom. When many water molecules are near one another, the partially positive hydrogen atoms of some molecules are attracted to the partially negative oxygen atoms of neighboring water molecules. These attractions are hydrogen bonds, weak electrical attractions.