

 DRIVING QUESTIONS
DRIVING QUESTIONS
- What is the structure of tissues and organs, and how can organs be repaired or replaced?
- What are the properties of specialized cells in tissues, and how do stem cells differentiate into these specialized cells?
- How do stem cells contribute to regenerative medicine, and how can we obtain or produce stem cells for this purpose?
In-Class Activity
Click here to access Lecture ppt specifically designed for chapter 13.
Click here to access Clicker Questions specifically designed for chapter 13.
 hen Luke Masella was 10 years old, he was in and out of the hospital nearly every week. Doctors didn’t know what was wrong with him. Toxins were building up in his blood, and he had lost a quarter of his body weight. After a multitude of tests, the doctors realized that Luke was in kidney failure and traced the problem to a faulty bladder. Urine was backing up inside the kidney and making it malfunction.
hen Luke Masella was 10 years old, he was in and out of the hospital nearly every week. Doctors didn’t know what was wrong with him. Toxins were building up in his blood, and he had lost a quarter of his body weight. After a multitude of tests, the doctors realized that Luke was in kidney failure and traced the problem to a faulty bladder. Urine was backing up inside the kidney and making it malfunction.
In 2001, Luke became one of the first patients in the world to receive an experimental treatment for such bladder problems: an engineered human bladder, one grown from his own cells, was implanted to fix his faulty one. “They take a piece of your bladder out. They grow it in a lab for 2 months into a new bladder that’s your own. And they put it back in,” Luke explained on ABC television in 2012.
This organ-growing technique is the brainchild of Anthony Atala, director of the Wake Forest University Institute for Regenerative Medicine. In 2006, Atala announced that he and his colleagues had successfully transplanted engineered human bladders into several children and teenagers, surprising the medical community. Although scientists had for years been transplanting organs like hearts and kidneys, the bladders were the first transplanted organs made with a person’s own cells.
“It was very significant work,” William Wagner, deputy director of the McGowan Institute for Regenerative Medicine at the University of Pittsburgh, says of Atala’s accomplishment. “He’s overcome a huge number of challenges.”
The potential applications of the technique are enormous. Each year, the demand for transplant organs such as hearts, livers, and kidneys vastly exceeds supply. In 2012, for example, surgeons transplanted about 30,000 organs, according to the Organ Procurement and Transplantation Network. Meanwhile, there are about 100,000 people waiting for an organ transplant. And even when an organ does become available, the recipient’s body may reject the organ because the donor and recipient immune systems are not compatible–leaving the patient sicker than before the transplant.
 They take a piece of your bladder out. They grow it in a lab for 2 months into a new bladder that’s your own. And they put it back in.
They take a piece of your bladder out. They grow it in a lab for 2 months into a new bladder that’s your own. And they put it back in.
–LUKE MASELLA
STEM CELLS Immature cells that can divide and differentiate into specialized cell types.
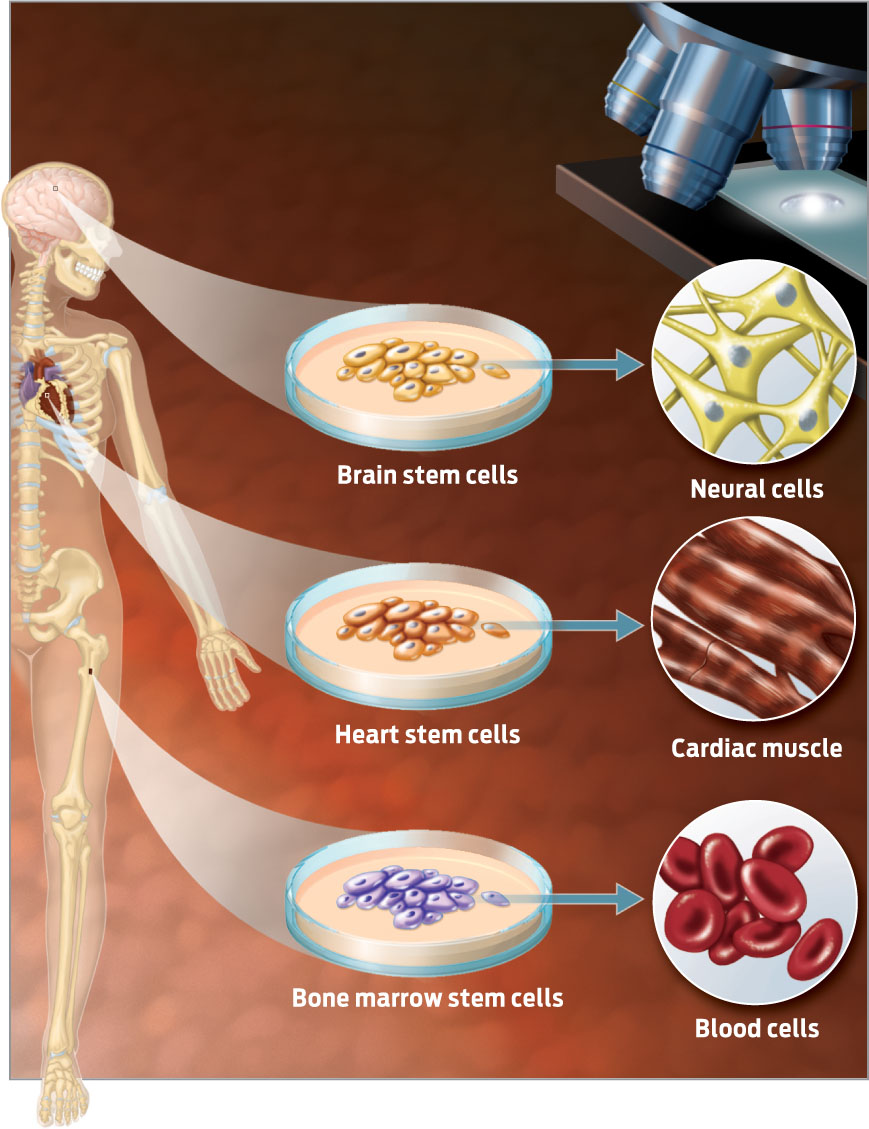
Growing organs from a person’s own cells not only sidesteps organ rejection, it also eliminates the need for donors. A decade ago, most scientists considered such a feat a pipe dream. After all, many human organs are complex three-dimensional structures made up of millions of cells–how would scientists manage to build such a structure by hand? But since the 2000s, advances in our understanding of stem cells have brought this fantasy closer to reality. Stem cells are immature cells that divide repeatedly and give rise to more-specialized cell types. They are important for development, growth, and repair of the body. Stem cells in the bone, heart, and brain, for example, help to regenerate those tissues and organs (INFOGRAPHIC 13.1).
Stem cells in various tissues divide to produce more stem cells and the specialized cells that make up that tissue. In this way, stem cells help keep the tissues in which they reside healthy.
With their regenerative properties, stem cells may hold the key to healing damaged or diseased body parts–they can do much of the construction work themselves. Already, scientists are using stem cells to construct new organs for transplant. One particularly eye-catching method is something dubbed “bioprinting,” a technique that uses computer graphics and cellular “ink” to manufacture organs from scratch. Need a new bladder? Just hit print.