REGENERATIVE MEDICINE
For scientists working to grow new organs, inspiration comes from an unlikely place: salamanders. These four-legged amphibians have a remarkable ability to regenerate body parts that have been injured or even severed completely. In presentations, Atala often shows a time-lapse video of a salamander growing back an entire front limb, complete with webbed digits.
If a salamander can regenerate tissues, Atala asks, why can’t a person? In fact, he says, they can. “The human body is constantly regenerating,” he explains. The whole superficial layer of our skin turns over every couple of weeks, he points out, while the cells lining our intestines turn over about every week. Even something as seemingly solid as bone is completely replaced about every 10 years. “Basically you don’t have any cells left over that were there 10 years prior,” Atala says (TABLE 13.1).
ADULT (SOMATIC) STEM CELLS Stem cells located in tissues that help maintain and regenerate those tissues.
Scientists have known for decades that some cells, like skin and blood cells, divide continually to repair and replace these tissues. But only within the past 20 years have they discovered that stem cells are found in many organs of the body: they are found in bladders, for example, and in many–perhaps all–tissues. Stem cells found in mature tissues are known as adult stem cells or somatic stem cells (“somatic” means “of the body”). Scientists are still searching for exactly where adult stem cells are located in various tissues. They suspect these cells reside in particular niches where they wait, not dividing, until disease or injury triggers them to divide.
Both humans and salamanders have adult stem cells. The main difference between humans and salamanders is that humans are genetically programmed to form scar tissue at the site of a wound, whereas salamanders grow new tissues. Scar tissue serves to protect us by sealing off a wound from the outside world, but the downside is that it interferes with regeneration. If there were some way to control scar formation in humans, researchers reason, further regeneration might be possible. “We have not lost the ability to regenerate,” Atala stresses. “The question is how can we harness that potential again?”
CELLULAR DIFFERENTIATION The process by which a cell specializes to carry out a specific role.
289
DIFFERENTIAL GENE EXPRESSION The process by which genes are “turned on,” or expressed, in different cell types.
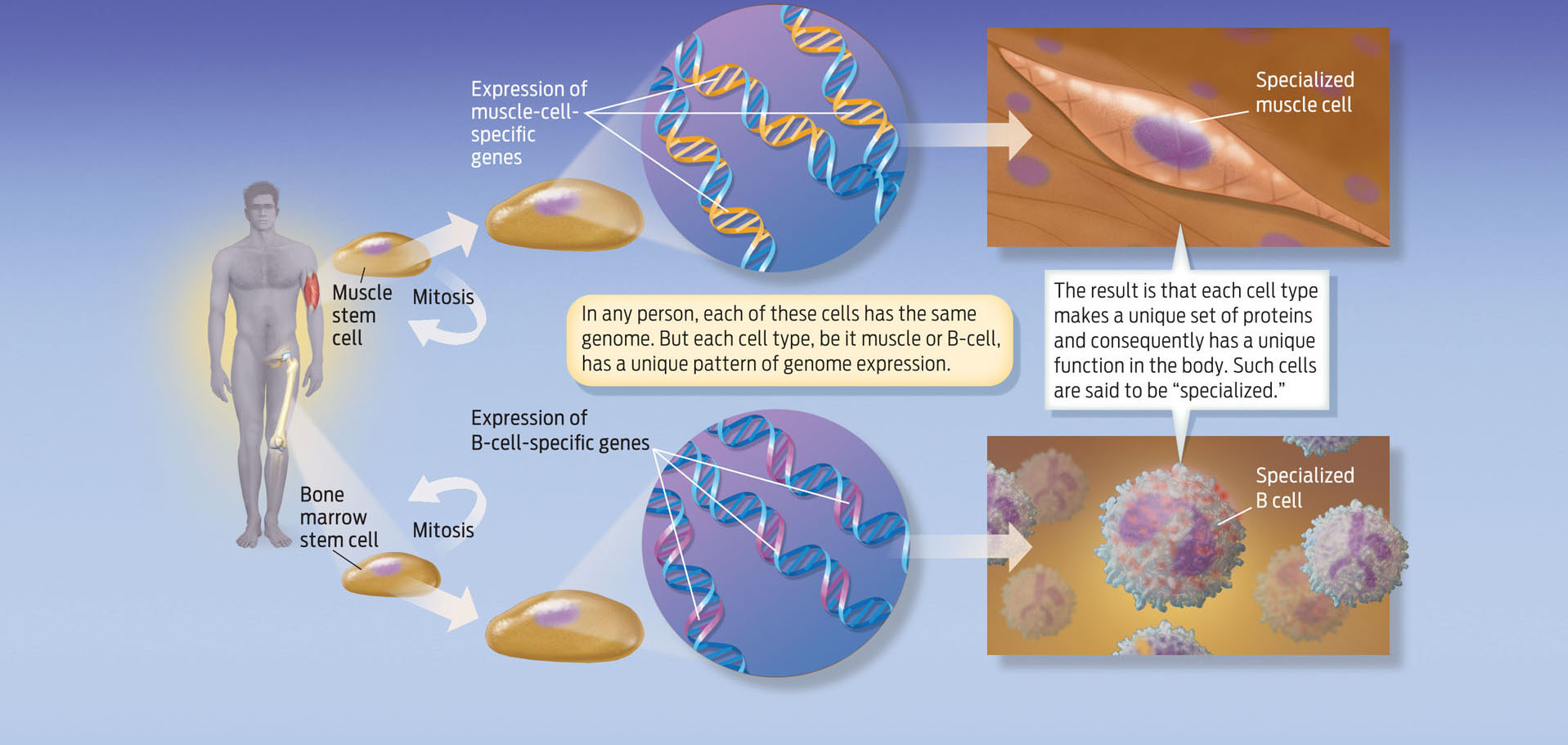
To heal tissue damaged by injury or disease, stem cells must do more than simply divide repeatedly. The new cells must also go through a process of specialization to develop into the specific cell types appropriate to the tissue in need of healing. Remember that during embryonic development a single cell becomes millions as the embryo grows. These dividing cells eventually become specialized as muscle cells, kidney cells, heart cells, and more than 200 other cell types in the body by the time we are born. This process, in which a cell develops from an immature cell type into a more specialized one, is called cellular differentiation. Cells become specialized by turning some genes “on” and others “off,” a process called differential gene expression. So while every cell in our body carries the exact same DNA, it is a cell’s pattern of gene expression–and therefore the proteins produced from those genes (see Chapter 8)–that defines it as one cell type or another.
Take, for example, two cell types with very distinct characteristics: muscle cells and B cells of the immune system. Muscle cells, which are long and slender, allow the body to move by contracting and relaxing. B cells are round, with antibody receptors protruding from their surfaces that detect foreign objects like viruses and bacteria, and thus help the body fight off infection. A cell’s physical shape and function are determined by the kinds of protein found within it. Muscle cells contain proteins that allow them to contract and cause body movement; B cells contain proteins that allow them to fight infection. Both muscle cells and B cells contain exactly the same DNA, and therefore contain the same genes. But only a subset of those genes is turned on in each cell type. As a result, each cell type produces a unique set of proteins that distinguish one cell type from another (INFOGRAPHIC 13.4).
Every cell in your body has the same genes, or genome. What distinguishes one cell type from another is the pattern of gene expression and, consequently, the proteins each cell makes. A muscle cell makes a different set of proteins than a B cell, a type of immune-system cell. Muscle cells, for example, express large amounts of actin and myosin proteins, which help muscles contract, whereas B cells express high levels of antibody proteins, which help the body fight infections.
290
Some fully differentiated cells do not divide. As cells become more mature and specialized–becoming, for example, cardiac muscle cells or nerve cells–they lose the ability to undergo cell division, or they divide less frequently. Stem cells, on the other hand, retain the ability to divide almost indefinitely. When a stem cell divides, one of the daughter cells remains an immature stem cell, while the other one “grows up,” differentiating into a more specialized cell. In this way, stem cells contribute to the maintenance of tissues, as daughter cells replace cells that have reached the end of their life span, while also retaining the ability to supply additional cells in the future.
When scientists discovered that many tissues had their own pool of stem cells, the search began for ways to stimulate these stem cells to divide and differentiate when they otherwise would not, and thus help to repair tissues and organs. This field of research is called regenerative medicine.
The idea of using stem cells to regenerate damaged or diseased tissues isn’t entirely new. Doctors have been treating leukemia–a type of white blood cell cancer–with stem cell transplants for decades. Like other blood cells, white blood cells, called leukocytes, are derived from stem cells in the bone marrow. Leukemia is caused by defective leukocytes that divide uncontrollably. To treat leukemia, doctors administer chemotherapy to kill the patient’s bone marrow cells and then replace those cells with marrow cells from an immune-matched donor. Stem cells in the new marrow repopulate the patient’s bloodstream with healthy blood cells. The goal of regenerative medicine is similar: scientists want to use stem cells to heal damaged or diseased tissues. The difference is that regenerative medicine seeks to prod stem cells to divide and differentiate when they normally wouldn’t, stimulating them to make cell types that they wouldn’t normally make.
291
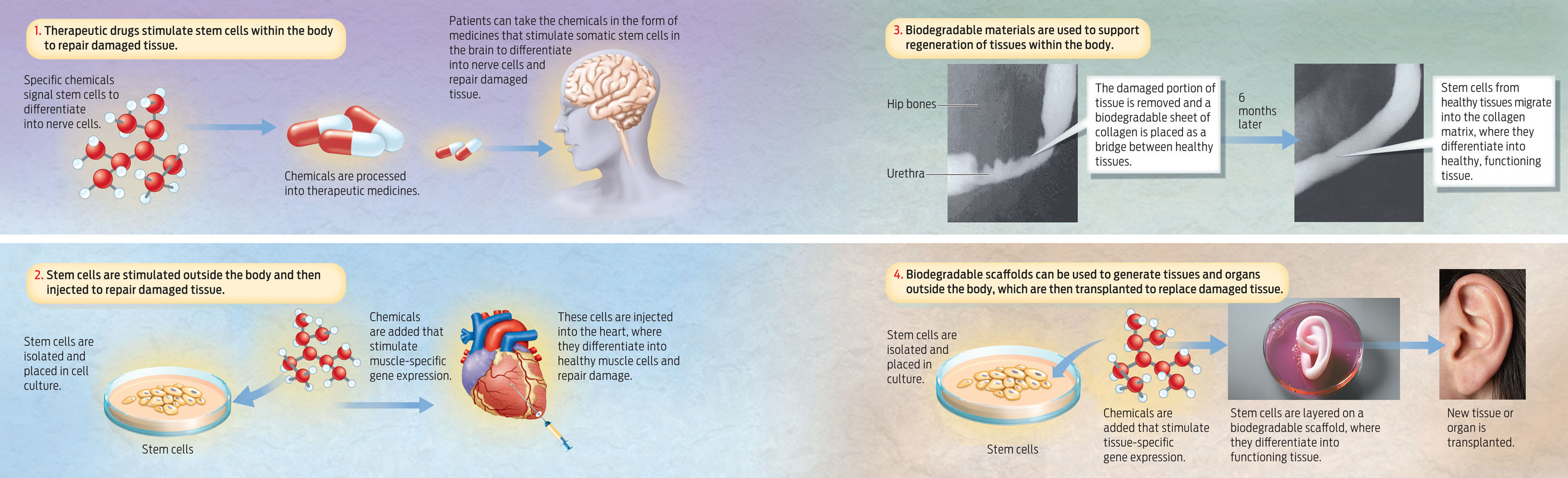
One approach in regenerative medicine would be the use of therapeutic drugs to stimulate specific stem cells in the body to grow and differentiate into the specialized cell types of the tissues that need repairing. Another approach would involve removing stem cells from the body, chemically inducing them to reproduce and differentiate, and then re-implanting the cells into a patient with a damaged tissue or organ.
A third approach, already in use, involves implanting the body with biodegradable scaffolds that encourage new tissue growth. Atala’s group has shown, for example, that stem cells can repair damaged urethras from inside the body. Give these cells a urethra-shaped scaffold to grow on, and new cells will migrate to the scaffold and regenerate a fully functioning urethra.
The fourth approach is one that Atala has used to create new bladders, ears, and other organs: seeding cells on a biodegradable scaffold outside the body, growing a new organ, then implanting it in a patient (INFOGRAPHIC 13.5).
292