PICKING UP, DROPPING OFF
HEMOGLOBIN A protein found in red blood cells specialized for transporting oxygen.
HEME GROUP Iron-containing structures on hemoglobin, the sites of oxygen binding.
If an athlete experiences hypoxia regularly, for 12–20 hours a day, for at least 3 weeks, according to the research he or she will start to see benefits upon returning to sea level. The biggest benefit will be the ability to carry more oxygen in the blood. But unlike carbon dioxide, which travels easily in the blood plasma as carbonic acid, oxygen does not readily dissolve in the watery plasma. How, then, does oxygen make its way through the blood? Oxygen is carried inside RBCs, bound to a molecule called hemoglobin. In fact, RBCs are essentially bags of hemoglobin. They lack a nucleus, and are streamlined for doing one thing very well: carrying oxygen.
Hemoglobin is a highly dexterous molecule, composed of multiple interworking parts. The oxygen-binding part is the iron-containing heme group. Each hemoglobin molecule has four heme groups, so each hemoglobin molecule can carry up to four molecules of O2. (Note that because the heme group of hemoglobin contains iron, a dietary deficiency of iron can limit the production of hemoglobin, and therefore reduce the overall oxygen-carrying capacity of blood, leading to a condition known as iron-deficient anemia.)
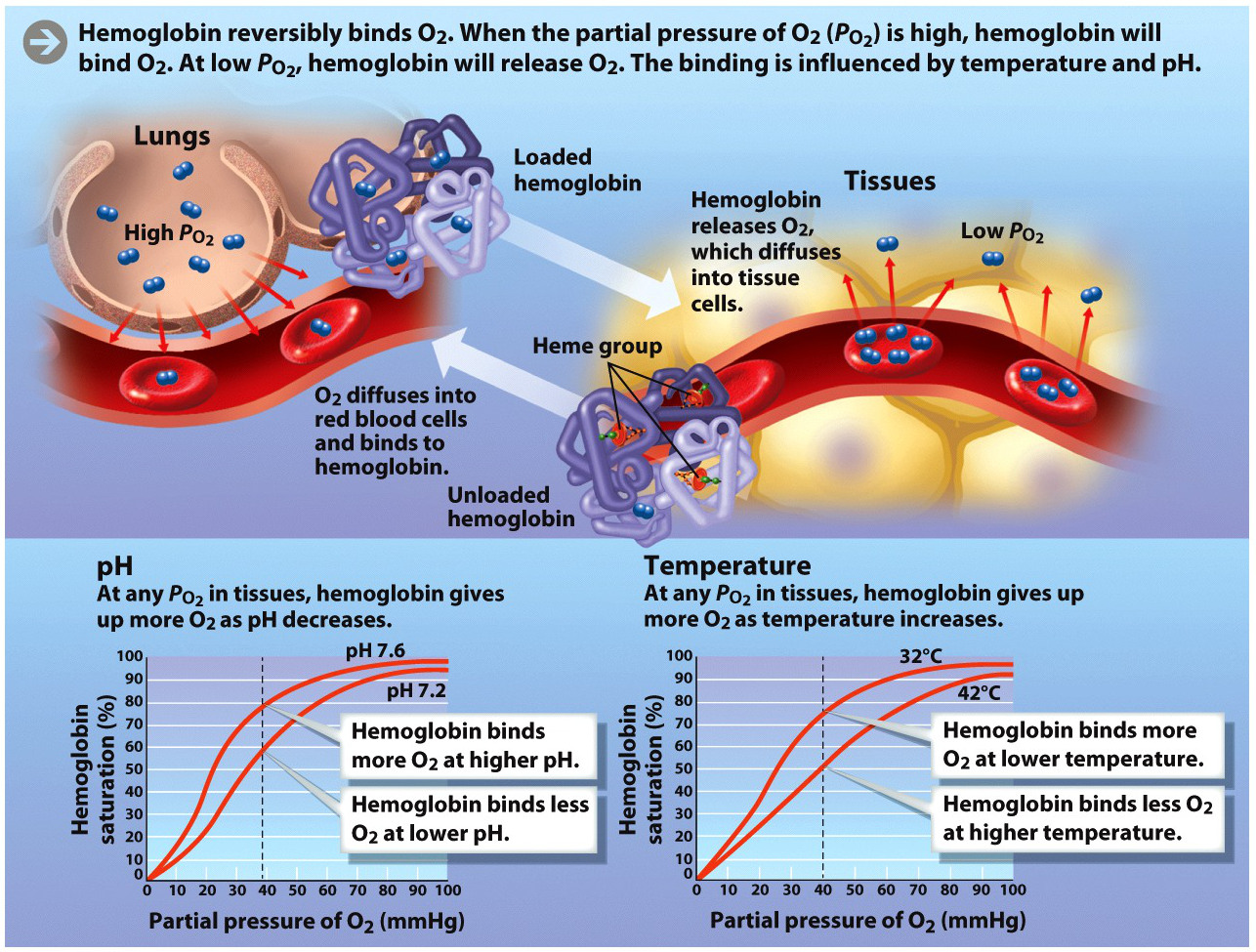
Key to understanding how hemoglobin functions in oxygen transport is the fact that it binds oxygen reversibly. It can “pick up” (bind) oxygen in the lungs and “drop off” (release) oxygen in the tissues.
What determines whether or not hemoglobin is picking up or dropping off oxygen, and therefore that oxygen is being delivered to the right place at the right time? There are a few important factors. The main one is the partial pressure of oxygen (![]() ). Fresh air entering the lungs has a relatively high partial pressure of oxygen, so the hemoglobin will become saturated with oxygen. In tissues, the partial pressure of oxygen tends to be lower, because cells in tissues are consuming oxygen as they carry out aerobic respiration, so hemoglobin tends to give up some of its oxygen to tissues.
). Fresh air entering the lungs has a relatively high partial pressure of oxygen, so the hemoglobin will become saturated with oxygen. In tissues, the partial pressure of oxygen tends to be lower, because cells in tissues are consuming oxygen as they carry out aerobic respiration, so hemoglobin tends to give up some of its oxygen to tissues.
Another important factor is temperature. Hemoglobin is more likely to release oxygen as temperature increases—as, for example, it does in an actively contracting muscle that is burning energy.
Lastly, pH plays a role. At lower pH, hemoglobin releases oxygen. As we’ve seen, one of the main things that can lower pH is the concentration of CO2. Since CO2 is produced during aerobic respiration, the pH of muscle goes down, becoming more acidic during exercise. In turn, the lower pH causes the hemoglobin to give up more of its oxygen, thus ensuring a continuous supply of the critical gas for the active muscle (INFOGRAPHIC 28.8).
So hemoglobin has an extremely important role to play in respiration. If there were some way to increase the amount of hemoglobin in blood, then an athlete would be at a competitive advantage in terms of conducting aerobic respiration.