SAM I AM
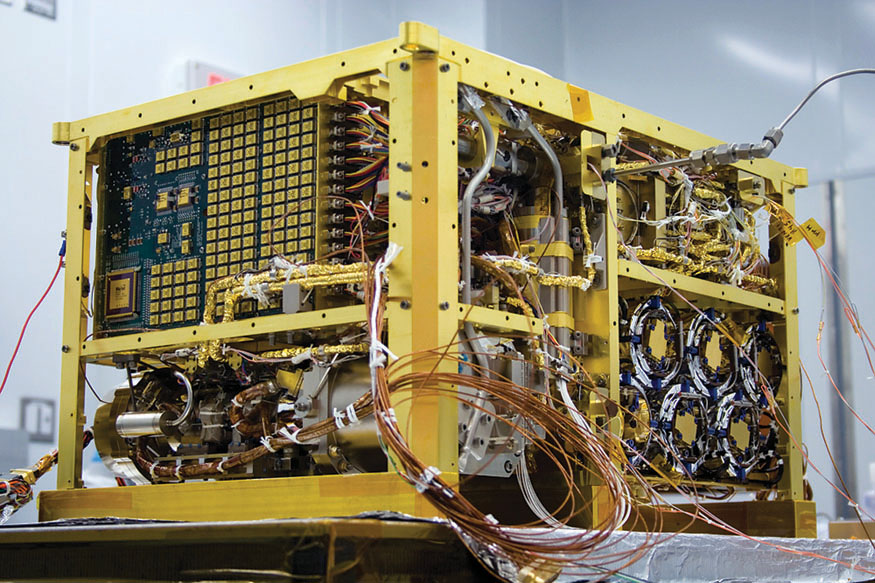
In his lab at NASA’s Goddard Space Flight Center in Greenbelt, Maryland, chemist Paul Mahaffy monitors a microwave-size device hairy with cords and wires. When it boots up, the device declares: “Sam I am, I am Sam!” in a playful homage to Dr. Seuss. SAM stands for “Sample Analysis on Mars.” It is the tool that Curiosity will use to check for life’s chemical building blocks. Mahaffy is SAM’s principal investigator.
SAM is mounted on Curiosity like a backpack. If the powerful cameras on board Curiosity are its eyes, then SAM is its extremely sensitive nose. And, according to Mahaffy, the instrument is “getting ready to start sniffing.”
COVALENT BOND A strong chemical bond resulting from the sharing of a pair of electrons between two atoms.
The sniffing takes place in a series of interconnected chambers. Once a sample of rock or dirt is scooped up by Curiosity, it is popped into SAM, where it is baked to a high temperature. The gases given off from this high-tech crockpot are then analyzed to determine their precise chemical makeup. The whole operation takes about a day, but interpreting the results can take much longer.
MOLECULE Atoms linked by covalent bonds.
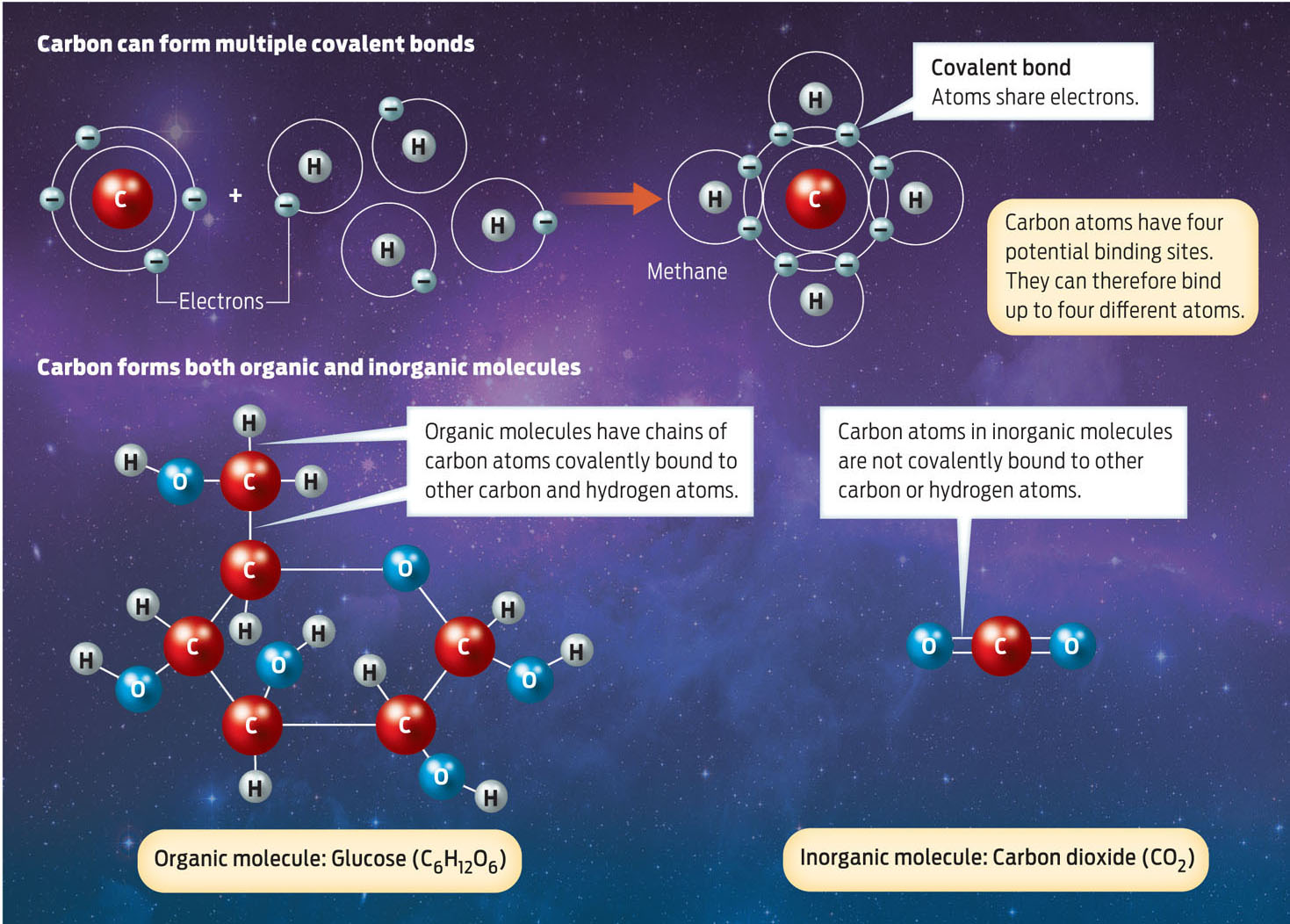
According to Mahaffy, SAM is particularly curious about the carbon on Mars–does it appear linked to other carbon atoms in chains or rings, as it is in living things on Earth? One way that atoms link is by sharing electrons to form a covalent bond between them. Atoms linked by covalent bonds form molecules. Different atoms can form different numbers of covalent bonds, in different geometries. Carbon atoms have four attachment, or bonding, sites, giving the element enormous versatility in forming molecules.
ORGANIC Describes a molecule with a carbon-based backbone and at least one C-H bond.
Living things on Earth are made up of so-called organic molecules, which have a backbone of interconnected carbon atoms and at least one carbon attached to a hydrogen atom. Most organic molecules require living things to make them, which is why they are often telltale signs of life. An example of a simple organic molecule is glucose, a type of sugar. Its molecular formula is C6H12O6. This means that each molecule of glucose has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Glucose is a ring-shaped molecule, with the carbon atoms forming the backbone of the ring.
INORGANIC Describes a molecule that lacks a carbon-based backbone and C-H bonds.
Nonliving things can also contain carbon, but this carbon is inorganic: inorganic molecules do not have a carbon-carbon backbone and a carbon-hydrogen bond. Carbon dioxide (CO2), for example, is an inorganic molecule, one found in the atmospheres of both Mars and Earth (INFOGRAPHIC 2.4).
CARBOHYDRATE An organic molecule made up of one or more sugars. A one-sugar carbohydrate is called a monosaccharide; a carbohydrate with multiple linked sugars is called a polysaccharide.
PROTEIN An organic molecule made up of linked amino acid subunits.
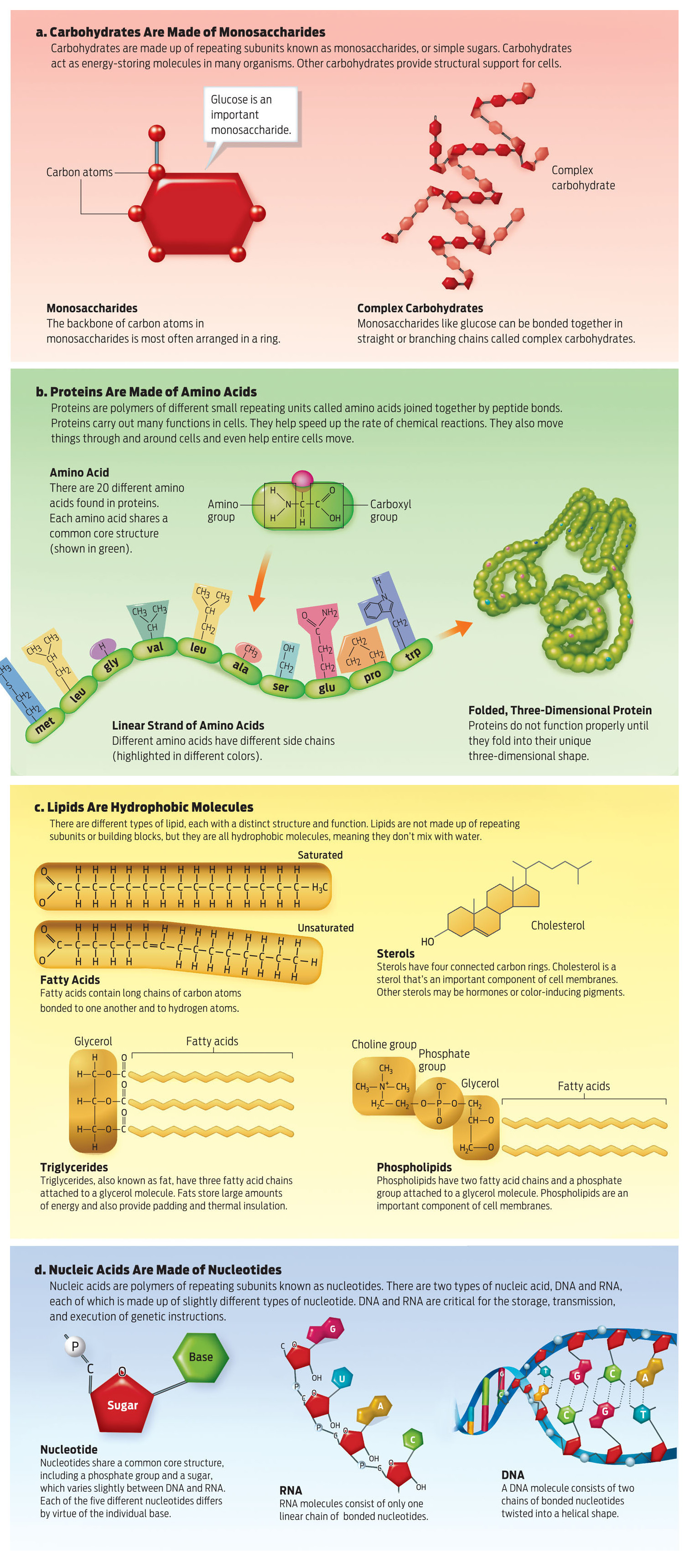
Just four types of organic molecules make up living things on Earth: carbohydrates, proteins, lipids, and nucleic acids. Every molecule in the human body can be classified as one or other of these organic molecules. Your skin, for example, is composed of the proteins collagen and elastin, and the protein hemoglobin carries oxygen in your blood. The padding in your soft spots is composed of lipids called triglycerides, also known as fats. And in your liver and muscle cells, a carbohydrate called glycogen helps store energy. All of these organic molecules have a backbone of interlinked carbon atoms.
LIPIDS Organic molecules that generally repel water.
NUCLEIC ACIDS Organic molecules made up of linked nucleotide subunits; DNA and RNA are examples of nucleic acids.
MACROMOLECULES Large organic molecules that make up living organisms; they include carbohydrates, proteins, and nucleic acids.
MONOMER One chemical subunit of a polymer.
Organic molecules can be quite large and are therefore considered macromolecules. Macromolecules share a similar organization in that they are composed of subunits called monomers linked together in a chain. When two or more monomers join they form a polymer. Carbohydrates, for example, are polymers made up of linked monomers called monosaccharides; similarly, proteins are made up of subunits called amino acids that are bonded together; and nucleic acids are polymers composed of nucleotides that form long chains (see UP CLOSE: MOLECULES OF LIFE).
POLYMER A molecule made up of individual subunits, called monomers, linked together in a chain.
MONOSACCHARIDE The building block, or monomer, of a carbohydrate.
AMINO ACID The building block, or monomer, of a protein.
NUCLEOTIDE The building block, or monomer, of a nucleic acid.
UP CLOSE MOLECULES OF LIFE: CARBOHYDRATES, PROTEINS, LIPIDS, NUCLEIC ACIDS

Using SAM’s powerful sensors, Curiosity will test Martian soil for these molecules of life or their subunits. While their presence would not absolutely prove the existence of life-a few can be made without life-it would show that Mars has one of the main ingredients necessary for building life. So it is an important first step.
As for what organic molecules, or “organics,” he hopes to find, McKay isn’t picky. Just to go from no organics to organics, he says, would be an exciting first step. Once scientists locate the organics, they can take the next logical step and ask whether they resemble ones on Earth: “Are any of them sugars? Or amino acids? Or chocolate?” he says. That’s when things will really get interesting.
McKay cautions that recognizing evidence of life might be harder than we realize. After all, he says, we have only one example of life-Earth life-so we have to be careful about extrapolating. Nevertheless, the fact that Mars is so close to Earth, and that the early histories of the two planets were likely very similar, means that any remnants of life on Mars might conceivably resemble those on Earth.
They may even share a common origin.