CHAPTER 2 Summary
In-Class Activity
Click here to access the Test Banks specifically designed for chapter 2.
 Life is difficult to define in universal terms because we have only a single example of it to consider: life on Earth.
Life is difficult to define in universal terms because we have only a single example of it to consider: life on Earth. On Earth, living organisms share a number of functional characteristics: they grow and reproduce, maintain homeostasis, sense and respond to their environment, and rely on energy to carry out their functions.
On Earth, living organisms share a number of functional characteristics: they grow and reproduce, maintain homeostasis, sense and respond to their environment, and rely on energy to carry out their functions. All matter is composed of elements, of which there are about 100 in the universe. Each element has a unique atomic structure, with a particular number of protons, neutrons, and electrons.
All matter is composed of elements, of which there are about 100 in the universe. Each element has a unique atomic structure, with a particular number of protons, neutrons, and electrons. When atoms share pairs of electrons they form covalent bonds, building molecules.
When atoms share pairs of electrons they form covalent bonds, building molecules. On Earth, living organisms are made up of organic molecules, those containing a backbone of the element carbon.
On Earth, living organisms are made up of organic molecules, those containing a backbone of the element carbon. Four types of carbon-based organic molecule make up living things: proteins, carbohydrates, nucleic acids, and lipids.
Four types of carbon-based organic molecule make up living things: proteins, carbohydrates, nucleic acids, and lipids. Living organisms on Earth are made of cells, which contain water and are surrounded by a cell membrane; cells are the smallest unit of life.
Living organisms on Earth are made of cells, which contain water and are surrounded by a cell membrane; cells are the smallest unit of life. Water is a polar molecule, with a partial positive and a partial negative charge.
Water is a polar molecule, with a partial positive and a partial negative charge. Because water molecules have partially positive hydrogen atoms and partially negative oxygen atoms, they can form hydrogen bonds (attractions between these opposite partial charges) with each other and interact with other charged molecules.
Because water molecules have partially positive hydrogen atoms and partially negative oxygen atoms, they can form hydrogen bonds (attractions between these opposite partial charges) with each other and interact with other charged molecules. Water has many properties that make it a crucial component of life on Earth: it is “sticky,” it regulates heat well, it floats when frozen, and it is a good solvent.
Water has many properties that make it a crucial component of life on Earth: it is “sticky,” it regulates heat well, it floats when frozen, and it is a good solvent. When atoms lose or gain electrons, they become ions. Oppositely charged ions can form ionic bonds—strong electrical attractions. Water is a good solvent of substances with ionic bonds.
When atoms lose or gain electrons, they become ions. Oppositely charged ions can form ionic bonds—strong electrical attractions. Water is a good solvent of substances with ionic bonds. Substances that, like salt, easily dissolve in water are considered hydrophilic; substances that do not dissolve in water, like lipids, are hydrophobic.
Substances that, like salt, easily dissolve in water are considered hydrophilic; substances that do not dissolve in water, like lipids, are hydrophobic.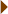 The concentration of H+ ions in a solution determines its pH. Most chemical reactions in cells take place at a nearly neutral pH.
The concentration of H+ ions in a solution determines its pH. Most chemical reactions in cells take place at a nearly neutral pH.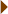 If life is found on other planets, it may or may not use the chemical framework used by life on Earth.
If life is found on other planets, it may or may not use the chemical framework used by life on Earth.MORE TO EXPLORE
 NASA, Mars Science Laboratory http://www.nasa.gov/mission_pages/msl/index.html
NASA, Mars Science Laboratory http://www.nasa.gov/mission_pages/msl/index.html
 Twitter @MarsCuriosity
Twitter @MarsCuriosity
 McKay, C. P. (2010) An origin of life on mars. Cold Spring Harbor Perspectives in Biology. march 3.
McKay, C. P. (2010) An origin of life on mars. Cold Spring Harbor Perspectives in Biology. march 3.
 McKay, C. P. (2004) What is life—and how do we search for it in other worlds? PLoS Biol 2(9):1260–1263.
McKay, C. P. (2004) What is life—and how do we search for it in other worlds? PLoS Biol 2(9):1260–1263.
 Cleland, C. E., and Chyba, C. F. (2007) Does ‘life’ have a definition? In Sullivan, W. T., III, and Baross, J. A., eds., Planets and Life: The Emerging Science of Astrobiology. Cambridge, UK: Cambridge University Press.
Cleland, C. E., and Chyba, C. F. (2007) Does ‘life’ have a definition? In Sullivan, W. T., III, and Baross, J. A., eds., Planets and Life: The Emerging Science of Astrobiology. Cambridge, UK: Cambridge University Press.
 The Limits of Organic Life in Planetary Systems. (2007) Washington, DC: National Academies Press. http://www.nap.edu/catalog.php?record_id=11919#toc
The Limits of Organic Life in Planetary Systems. (2007) Washington, DC: National Academies Press. http://www.nap.edu/catalog.php?record_id=11919#toc
Page 41