ENERGY BASICS
FOSSIL FUELS Carbon-rich energy sources, such as coal, petroleum, and natural gas, which are formed from the compressed, fossilized remains of once-living organisms.
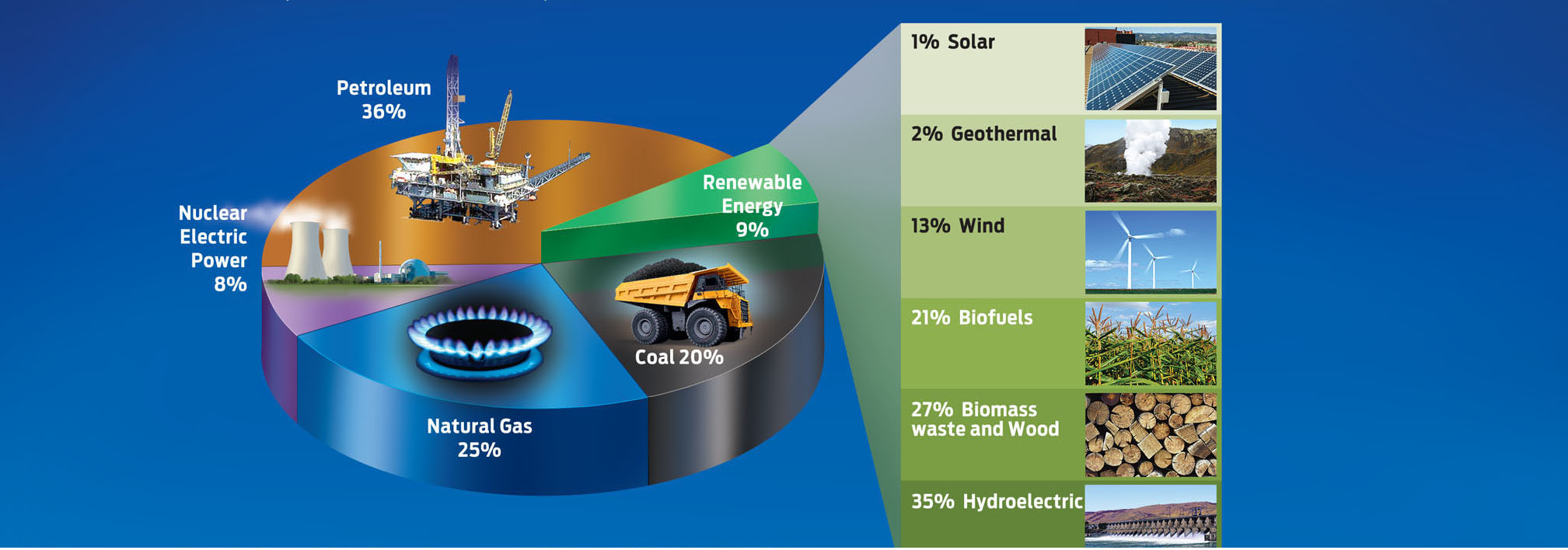
To power nearly everything in our modern lives we need energy. Americans get their energy from many sources, by far the largest of which are the fossil fuels—oil, gas, and coal. The compressed remains of once-living organisms, fossil fuels are considered to be nonrenewable because they take millions of years to form. Currently, only about 9% of our energy comes from renewable sources like wind, solar, and biofuels, but that proportion is growing. For powering our planes and cars, the most useful fuel sources are those that come in liquid form, such as gasoline and diesel, both derived from oil (petroleum) (INFOGRAPHIC 5.2).
The United States is the largest consumer of fossil fuels. Fossil fuels are considered non-renewable because they take millions of years to create by natural processes. As we continue to deplete fossil fuels, new energy sources are being developed that reduce our demand on petroleum and other fossil fuels.
ENERGY The capacity to do work. Cellular work includes processes such as building complex molecules and moving substances into and out of the cell.
CHEMICAL ENERGY Potential energy stored in the bonds of biological molecules.
Of course, energy isn’t just needed to fly planes and drive cars. Energy—defined as the capacity to do work—is critical to all life on earth. Energy powers every activity we perform, from the more obvious ones like breathing, thinking, and running to less obvious activities like building the molecules that make up our bodies. Without a source of energy, all life on Earth would grind to a halt, like a cell phone with a dying battery.
Organisms can’t simply create energy when they need it, however—energy cannot be created—they must obtain it from an outside source. Humans and other animals obtain the energy they need by eating food. We’ve already seen that our digestive systems break down food to obtain nutrients (see Chapter 4). As these molecules are further broken down, the energy stored in the molecules is made available to do work. The bonds that hold molecular subunits together represent a form of stored chemical energy; breaking these bonds releases that stored energy, making it available to power cell functions.
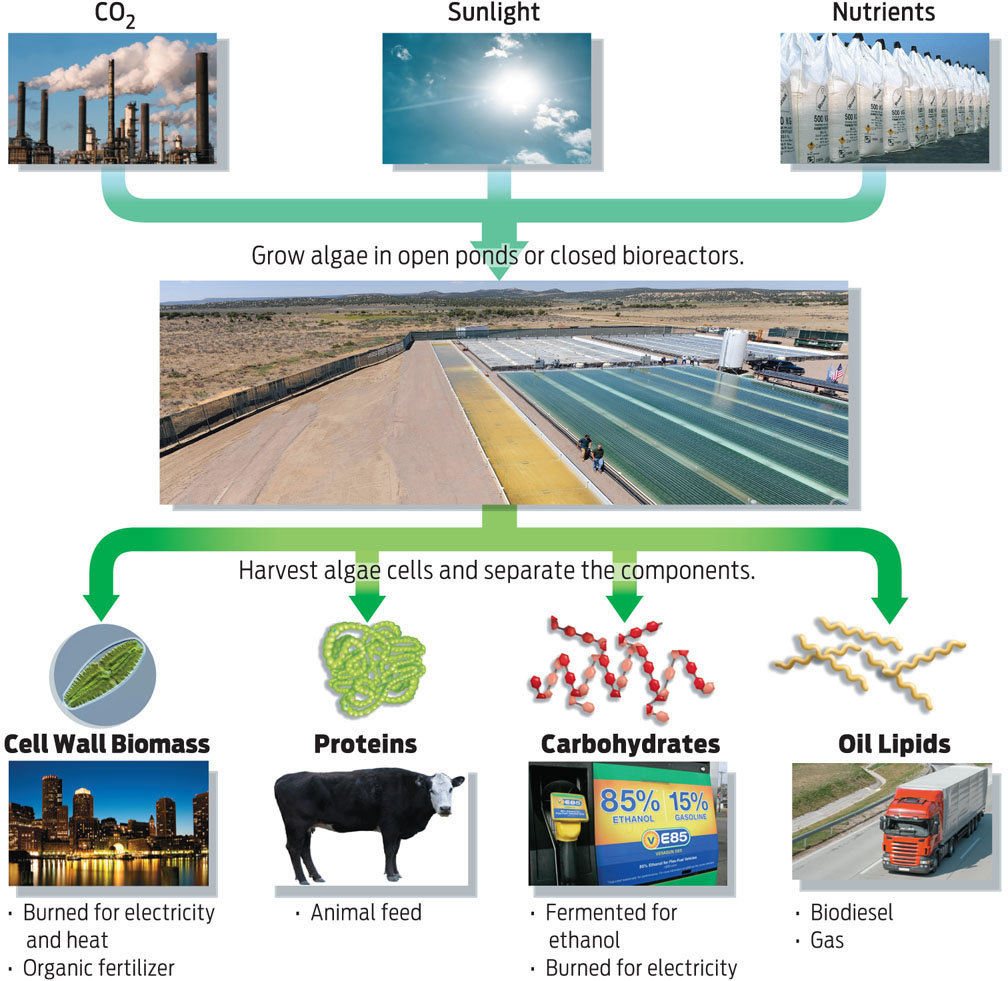
Algae and plants, on the other hand, get their energy from the sun. They trap the energy of sunlight and store it in the form of molecules inside their cells. Algae could be the world’s next energy source because these tiny organisms are very good at trapping and storing energy—the oil they produce is rich in chemical energy. Even more impressive, all they need to make this oil is sunlight, carbon dioxide, and water (plus a few nutrients). Give them these tidbits, and algae grow rapidly—some strains double their volume in 12 hours—all the while accumulating gobs of oil inside their cells. In addition to this oil, which can be used to make biodiesel, algae also make proteins and carbohydrates (sugars), which can be converted into other biofuels, like ethanol and butanol. These biofuels can be mixed with gasoline to power cars (INFOGRAPHIC 5.3).
Algae can use sunlight, carbon dioxide, and nutrients to produce a high volume of oil readily used to produce biofuel, in addition to carbohydrates and proteins that can be useful as additional energy sources.
CONSERVATION OF ENERGY The principle that energy cannot be created or destroyed, but can be transformed from one form to another.
POTENTIAL ENERGY Stored energy.
Sears wasn’t the first to consider algae’s fuel potential. In 1978—the same year Sears took his fateful night dive—the U.S. Department of Energy started its Aquatic Species Program, with the goal of exploring algae’s fuel possibilities. This venture was a direct response to the oil crisis of the 1970s, during which the cost of oil skyrocketed, supplies were rationed, and long lines formed at the pump. But when oil fell to $20 a barrel in 1996, the government abandoned the program, assuming that oil made from algae would always be too expensive. Now, with oil prices much more volatile, and the cost of alternatives coming down, biofuel from algae has become an attractive option again.
KINETIC ENERGY The energy of motion or movement.
With so much talk about dwindling energy reserves, it’s tempting to think that energy is something that we simply use up over time. But energy cannot be created or destroyed. When energy is used to power our cars—or our brain cells—that energy is not destroyed, it merely changes form, a principle known as the conservation of energy. This principle, one of the laws of thermodynamics, is key to understanding energy use—as applicable to cars as to people.
HEAT The kinetic energy generated by random movements of molecules or atoms.
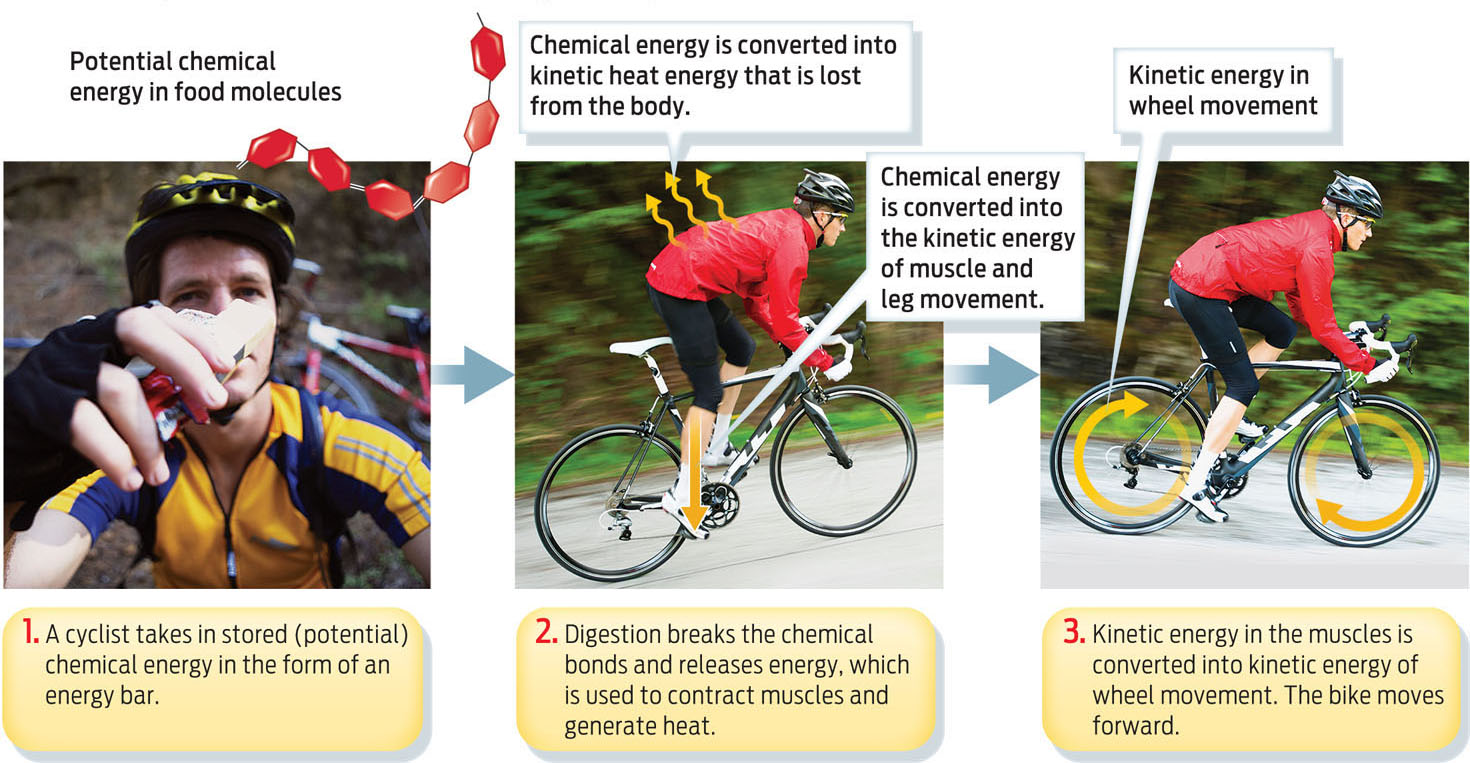
Consider a cyclist who eats a cereal bar before a ride. The bar contains chemical energy in the form of chemical bonds that hold the carbohydrate and protein molecules of that bar together. Chemical energy is potential energy, meaning that it is stored and waiting for use. When the cyclist eats and digests the bar, digestion breaks those chemical bonds, and the stored energy is released. As the cyclist begins to pedal, his body converts this potential energy into the kinetic energy of muscle contraction and heat. The kinetic energy of muscle movement is then converted into the kinetic energy of moving wheels. From start to finish, from cereal bar to spinning wheels, energy is converted from one form into another, but is never destroyed (INFOGRAPHIC 5.4).
Energy in the universe is neither created nor destroyed, but is converted from one form to another. Stored potential energy, for example, can be converted to kinetic energy, as the cyclist below illustrates.
Energy from biofuel has a similar life story. Oil from algae is rich in chemical energy: the lipids in the oil store energy in their bonds. When these bonds are broken—for example, when they are burned—they release large amounts of energy that can be used to power machines. In a car’s combustion engine, the chemical energy in biofuel is rapidly and explosively converted to heat energy that warms the gas molecules inside a chamber, causing them to expand. The expansion of the heated gas molecules pushes against the pistons, causing the wheels to move. The chemical potential energy of biofuel is thus converted into the kinetic energy of car movement.
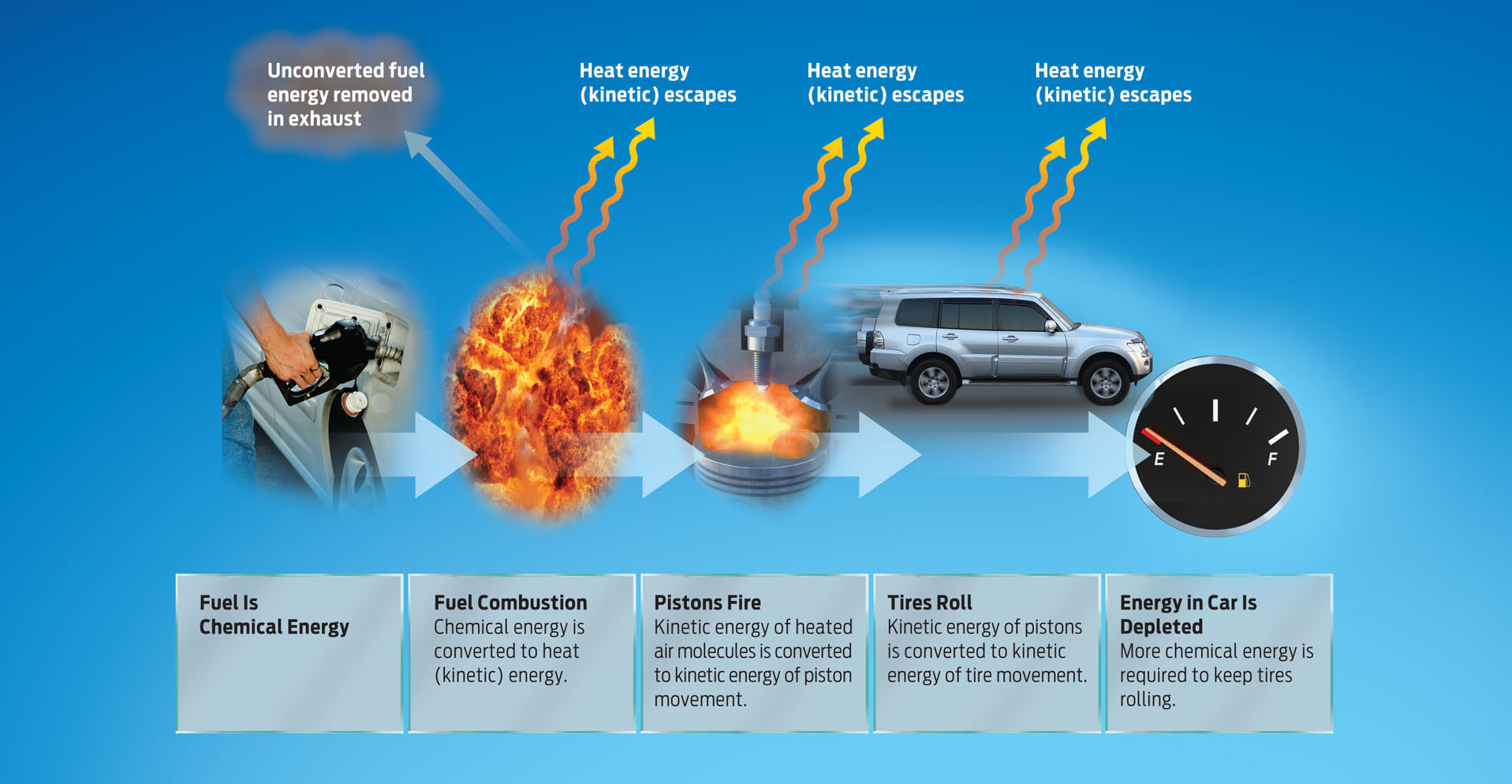
If energy is never destroyed, only converted, why do we need to keep filling our tanks? It turns out that the conversion of energy from one form to another isn’t 100% efficient. With each energy conversion, a bit of energy is “lost” to the environment as heat. This is why our bodies heat up when we exercise and why car engines are warm after being driven. In the case of a car engine, the generation of heat serves a purpose—to push the pistons. But heat lost to the outside of the car is wasted—it is no longer available to do useful work. This inefficiency is the reason we need to keep supplying energy to any system. We eat three meals a day to replenish the energy our bodies have lost as heat and converted into the chemical energy of cells and the kinetic energy of movement. Similarly, cars need a new tank of fuel after having burned through the previous one to convert the chemical energy of fuel into the heat and kinetic energy of motor movement. Just how well your car converts the chemical energy of gas into the kinetic energy of car speed determines your mpg—your miles per gallon. If an engine doesn’t combust efficiently, some of the fuel molecules will undergo chemical reactions and be converted to other molecules—like pollutants—rather than power the pistons as heat. If the pistons can’t use the heat efficiently, the heat will leave the car without powering the wheels. At each step of energy transformation, energy is lost from the car system and into the environment, and we’re back to the fuel pump once more (INFOGRAPHIC 5.5).
As energy is converted from one form to another, some of the available energy is not fully converted to the next form. Instead, some energy is converted into heat that escapes into the environment, and some energy is not converted at all.