Putting Food to Work
ADENOSINE TRIPHOSPHATE (ATP) The molecule that cells use to power energy-requiring functions; the cell’s energy “currency.”
Getting energy from food seems simple enough: we eat food and we have energy. But the process of releasing the energy stored in food and putting it to use in cells is a bit more complex. First, food must be broken down into its component subunits—among them, fats and sugars. Then, these breakdown products must go through a series of biochemical reactions that convert the chemical energy stored in these food molecules into a form of fuel we can use. Energy from food is ultimately captured in a molecule called adenosine triphosphate (ATP) that our cells use to carry out energy-requiring functions.
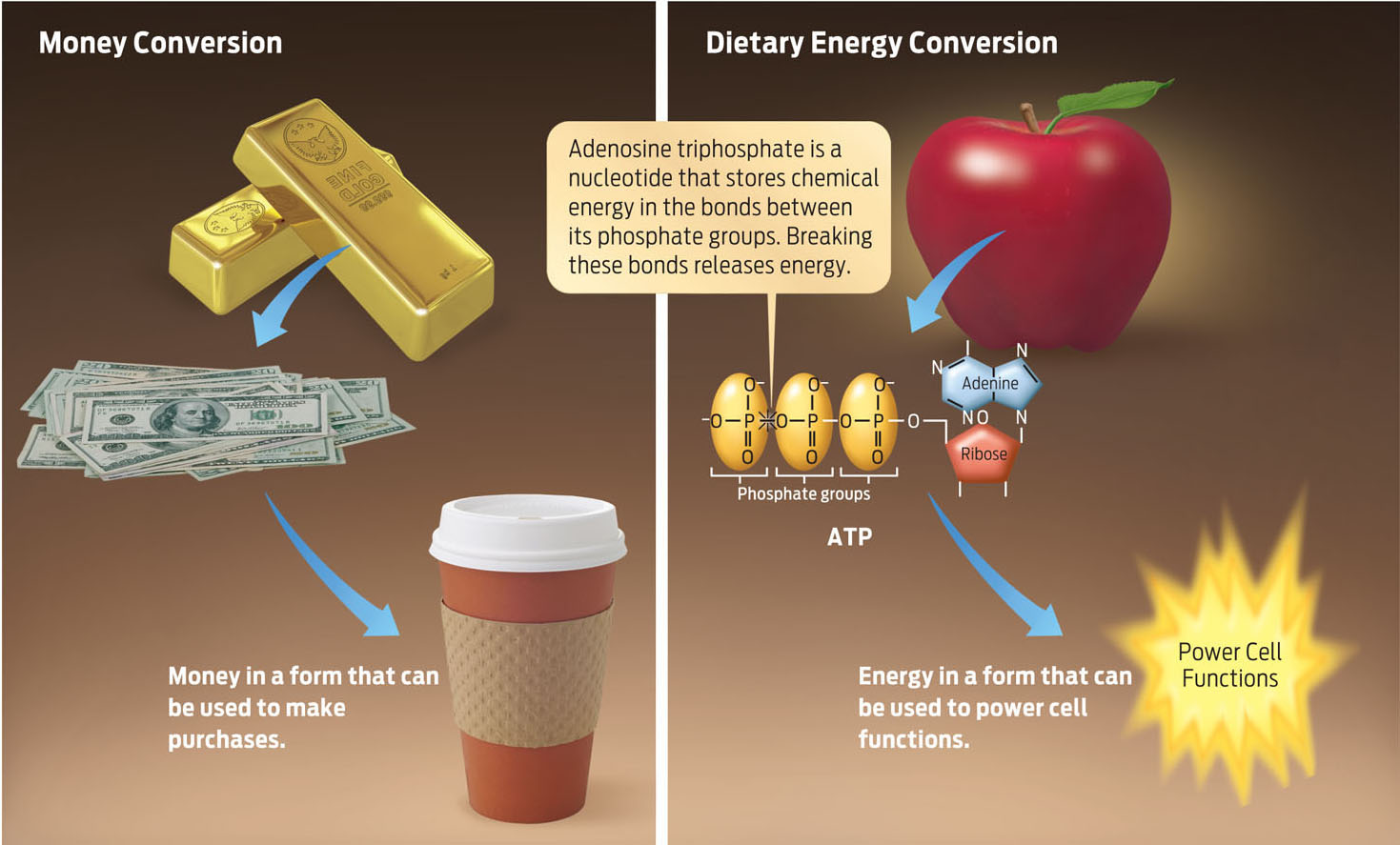
You can think of food as a bar of gold: it has a great deal of value, but if you carried that gold bar to your local convenience store, you wouldn’t be able to buy even a cup of coffee with it. You would first have to convert your gold bar into bills and coins. ATP is the energetic equivalent of bills and coins; it’s currency that your body can actually spend (INFOGRAPHIC 6.6).
Just as a gold bar must be converted to currency in order to buy merchandise, the energy in food must be converted to ATP before it can be used by the cell.
AEROBIC RESPIRATION A series of reactions that occurs in the presence of oxygen and converts energy stored in food into ATP.
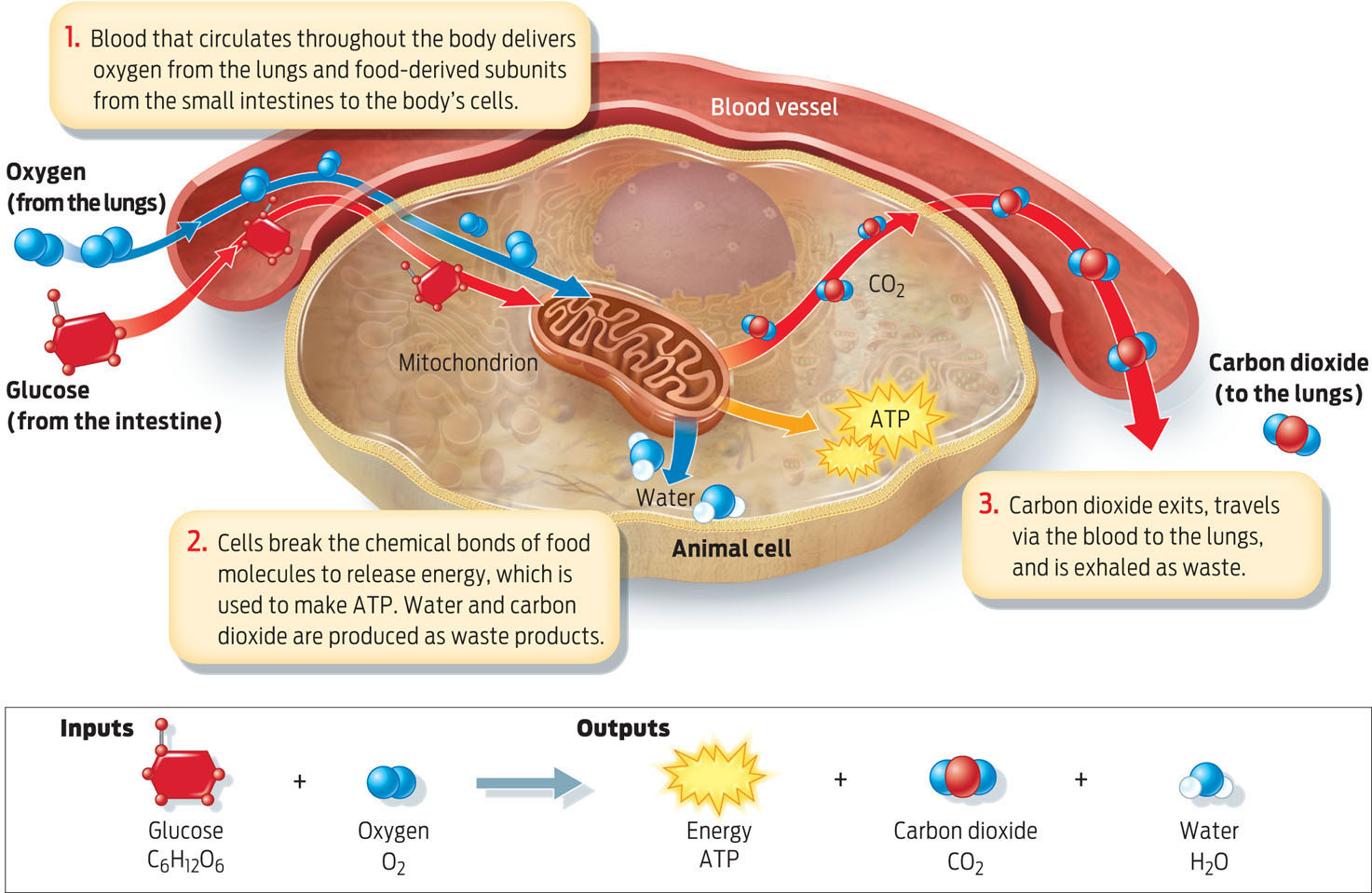
To make ATP, our bodies first break down food molecules into their smaller subunits through the process of digestion (see Chapter 26). Once released from food, such subunits as fatty acids, glycerol, amino acids, and sugars leave the small intestine and enter the bloodstream, which transports them to the body’s cells (see Infographic 6.4). Inside the cells, enzymes break apart the bonds holding these subunits together. The energy stored in those bonds is then captured and converted into the chemical bonds that make up ATP. When cells need energy, they tap these energy-rich bonds in ATP. When ATP bonds are broken, energy is released, allowing cells to “spend” their ATP currency and carry out normal cellular functions.
The primary process that all eukaryotic organisms, including plants, use to convert food energy into ATP is a form of cellular respiration called aerobic respiration. “Aerobic” means “in the presence of oxygen,” and this form of cellular respiration predictably requires a continual source of oxygen. Of the subunits released from food, glucose sugar is the most common source of energy for all organisms, from bacteria to humans. The aerobic respiration of glucose can be summarized by this equation:
Glucose + Oxygen → Energy + Carbon dioxide + Water
That is, the bonds holding the glucose molecule together are broken. Oxygen from the air we breathe is consumed in the process. When the bonds of glucose are broken, the energy is converted to ATP and some heat is given off as a result of the inefficiency of this conversion (see Chapter 5). Carbon dioxide and water are also given off as waste products of the process (INFOGRAPHIC 6.7).
During aerobic respiration, our cells use the oxygen we inhale to help extract energy from food. Cells convert the energy stored in food molecules into the bonds of ATP, the cell’s energy currency.
GLYCOLYSIS A series of reactions that breaks down sugar into smaller units; glycolysis takes place in the cytoplasm and is the first stage of both aerobic respiration and fermentation.
CITRIC ACID CYCLE A set of reactions that takes place in mitochondria and helps extract energy (in the form of high-energy electrons) from food; the second stage of aerobic respiration.
NAD+ An electron carrier. NAD+ can accept electrons, becoming NADH in the process.
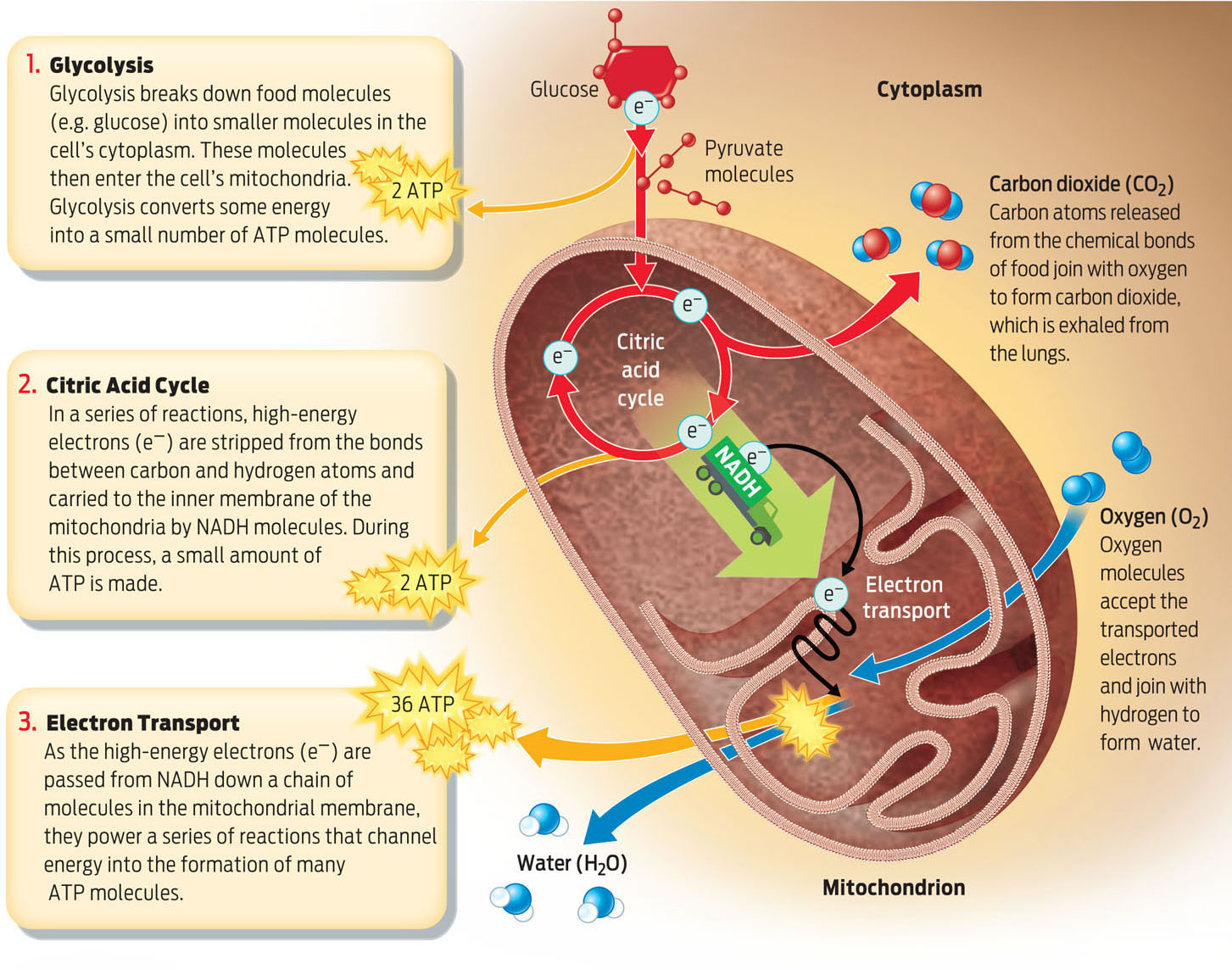
Aerobic respiration is a multistage process that takes place in different parts of the cell. The first stage, known as glycolysis, takes place in the cell’s cytoplasm. Glycolysis is a series of chemical reactions that splits glucose in half, into two smaller molecules of pyruvate. Each of these pyruvate molecules has three carbon atoms in its backbone. The pyruvate molecules then enter the cell’s mitochondria, where the next two stages of aerobic respiration occur.
ELECTRON TRANSPORT CHAIN A process that takes place in mitochondria and produces the bulk of ATP during aerobic respiration; the third stage of aerobic respiration.
During the second stage, the citric acid cycle, a series of reactions strips electrons from the bonds between the carbon and hydrogen atoms that were originally in glucose (and are now in pyruvate). The process releases CO2, which is ultimately exhaled from an organism’s lungs.
As the energy-rich bonds in glucose and pyruvate are broken, the electrons from those bonds are picked up by a molecule known as NAD+. When NAD+ picks up electrons, it becomes NADH (the electron-carrying form of the molecule). NADH then carries the electrons to the inner membrane of the mitochondria, where NADH gives them up (reverting to NAD+). The electrons then go through the third and last stage of aerobic respiration: the electron transport chain.
Electrons stripped from the bonds in glucose contain a lot of potential energy that is temporarily held by NADH. During electron transport, these energetic electrons are passed like hot potatoes from NADH down a chain of molecules in the inner mitochondrial membrane. Eventually the electrons are passed to oxygen molecules, which accept the electrons and combine with hydrogen atoms to produce water. As electrons pass down the chain, they supply the energy needed to form ATP. This electron transport chain fuels the bulk of ATP production (INFOGRAPHIC 6.8).
Nearly all eukaryotic organisms carry out aerobic respiration. The three main stages of aerobic respiration occur in specific locations within the cell and yield distinct products.
We’ve focused on glucose, but cells can also burn fats and amino acids for fuel during aerobic respiration. Because fats generally have more carbon-hydrogen bonds than do sugars and amino acids, they have more electrons to be stripped in the citric acid cycle. More electrons stripped means that more ATP molecules are produced during electron transport (which also explains why a gram of fat contains more Calories than a gram of sugar or protein).