MICROSCOPIC CLUES
In one sense, the idea of endosymbiosis was not entirely new. A few biologists in the late 19th century and early 20th century had suggested it after observing the remarkable resemblance between free-living bacteria and certain organelles of the eukaryotic cell. But in those early days it was not possible to test the idea, and so it was largely ignored. In 1925, Edmund Wilson, a prominent cell biologist, wrote, “To many, no doubt, such speculations may appear too fantastic for present mention in polite biological society.” But he went on to suggest that those speculations might “someday call for serious consideration.”
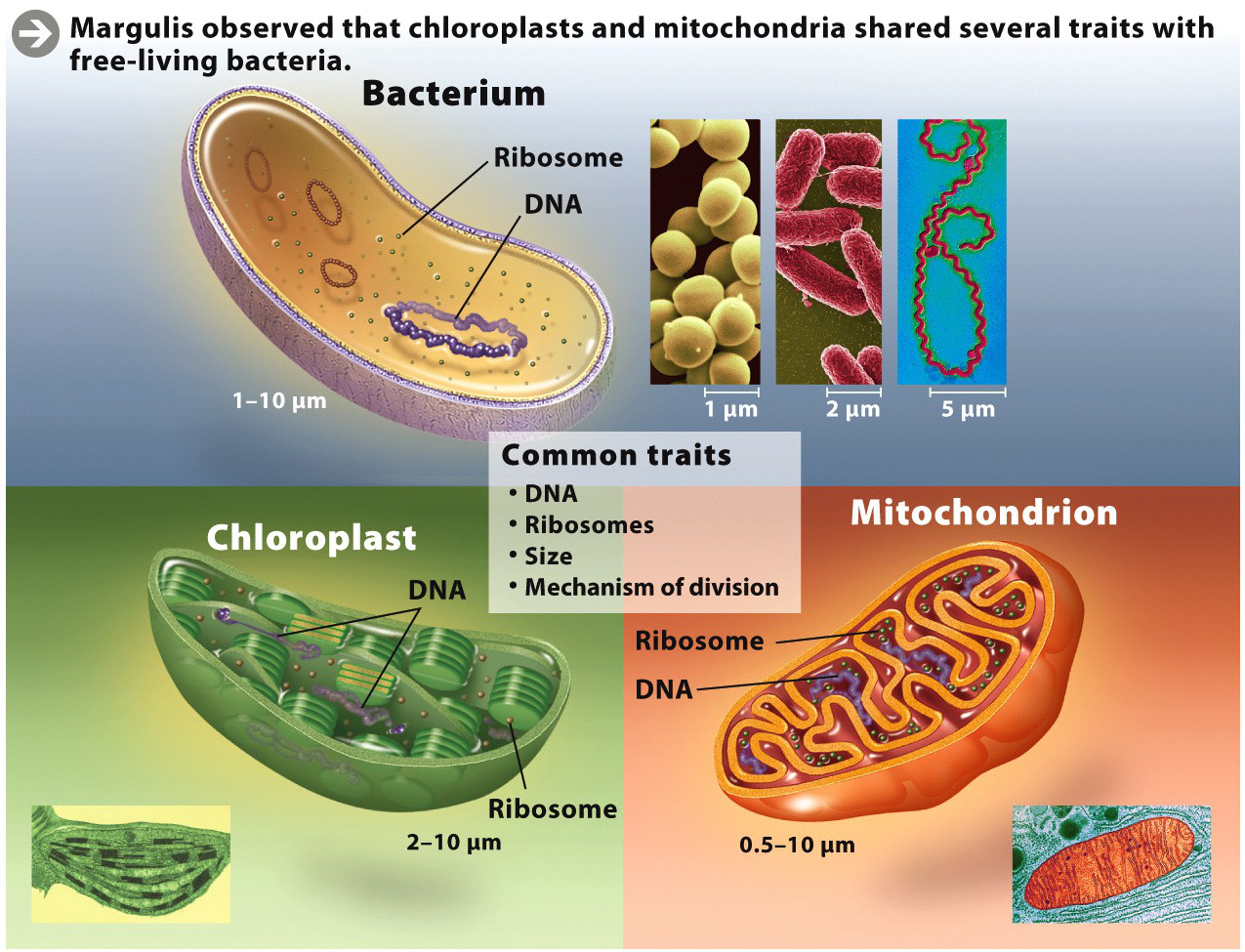
In 1960, while working on her master’s degree in biology at the University of Wisconsin, Margulis first became intrigued by the notion of endosymbiosis. Her advisors, Walter Plaut and Hans Ris, had recently made the startling discovery that chloroplasts–the tiny green organelles inside plant cells that carry out photosynthesis–had their own DNA, the molecule of heredity. Most DNA in a eukaryotic cell is housed in the nucleus, where it serves as the genetic blueprint for life. What was DNA doing in a chloroplast? To Margulis, the discovery suggested that chloroplasts had once led a separate existence as independent, free-living cells, and thus needed DNA to reproduce, much as bacteria do.
She pursued this idea further as part of her Ph.D. work at the University of California at Berkeley. She studied, in particular, the small unicellular eukaryote called euglena, which lives in water and contains numerous chloroplasts. She used radioactively labeled nucleotides, the building blocks of DNA, to show that the little squiggle inside a euglena chloroplast was indeed DNA, as her advisors had claimed. (Cells incorporate the nucleotides into DNA when they divide, and the radioactivity can be detected with photographic film.) This work lent additional support to the idea of endo-symbiosis.
The more Margulis looked, the more evidence she found. Not only did chloroplasts have their own DNA, but they also had ribosomes–the structures that, in the cytoplasm of both prokaryotic and eukaryotic cells, synthesize proteins. Chloroplasts were also about the same size as bacteria, and to reproduce they divided much the same way bacteria divide. Similarly, mitochondria, the cell’s “power plants,” also had these traits, suggesting that these organelles, too, had once been free-living cells (INFOGRAPHIC M1.1).
In her 1967 paper, Margulis gave credit to the biologists who had previously suggested endosymbiosis, but she did more than simply rehash old ideas. She brought together for the first time all the existing evidence from cell biology and biochemistry and wove it together into a coherent account. She also put the idea into evolutionary context and offered a rough timeline of when these events happened.
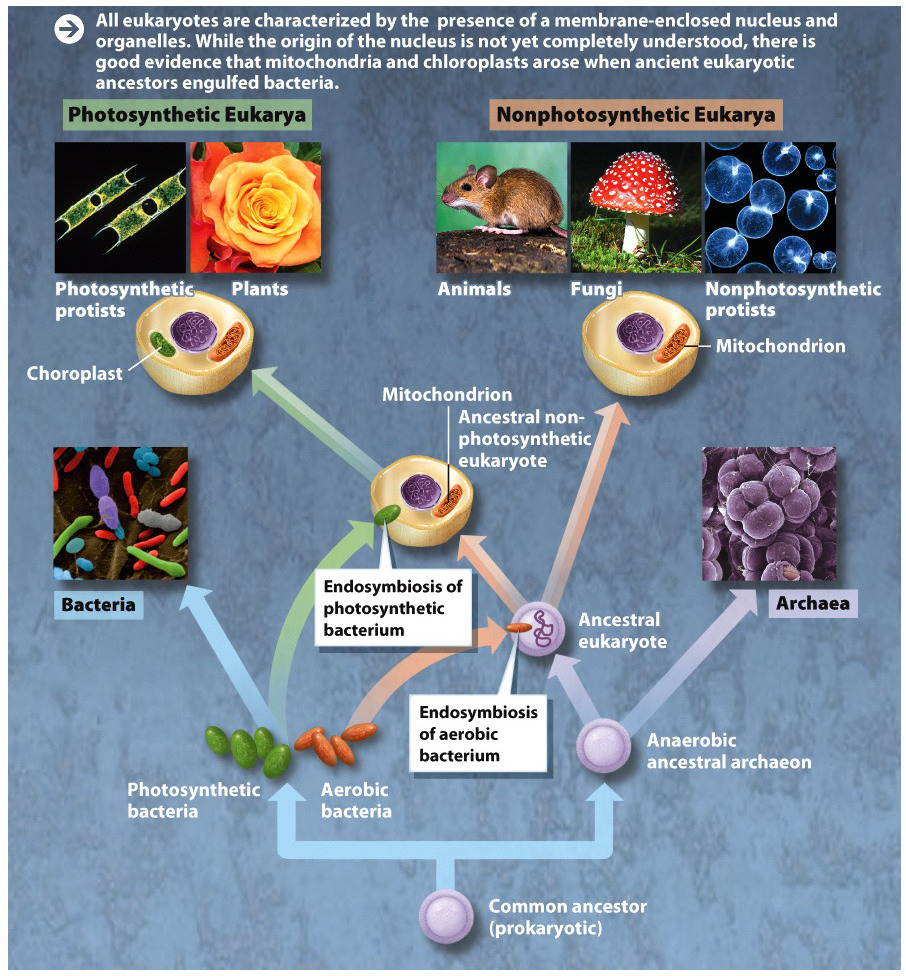
In Margulis’s view, the first cells on Earth were prokaryotic bacteria, first evolving some 4 billion years ago. These early cells were anaerobic–they did not use oxygen in their metabolism (which makes sense, since the early Earth had no substantial oxygen). These cells evolved and diversified for billions of years until, about 2.5 billion years ago, some developed the capacity to harvest the energy of sunlight, splitting water and releasing oxygen gas as a by-product. As oxygen built up in the atmosphere as a result of this photosynthesis, it produced an environment that strongly favored bacteria that could use oxygen in their metabolism.
At that point, according to Margulis, one such oxygen-using, or aerobic, bacterium was engulfed by a larger anaerobic cell. The ingested bacterium was not destroyed; instead, the two cells formed a symbiotic, mutually beneficial relationship. The smaller aerobic bacterium enabled the larger anaerobic cell to use oxygen to obtain energy, and the larger anaerobic cell provided the smaller bacterium with a source of sugars to eat. (These ideas bear a strong resemblance to the way that eukaryotic cells still, to this day, metabolize glucose during glycolysis and aerobic respiration; see Chapter 6.)
What began as a mutualistic relationship became, over time, an obligate one: the two cells could no longer survive apart, as each became increasingly codependent on the other. At some point later, this power duo got cozy with a third bacterium–a photosynthetic one–and engulfed it as well. These composite cells, Margulis argued, are the ancestors of eukaryotes (INFOGRAPHIC M1.2).
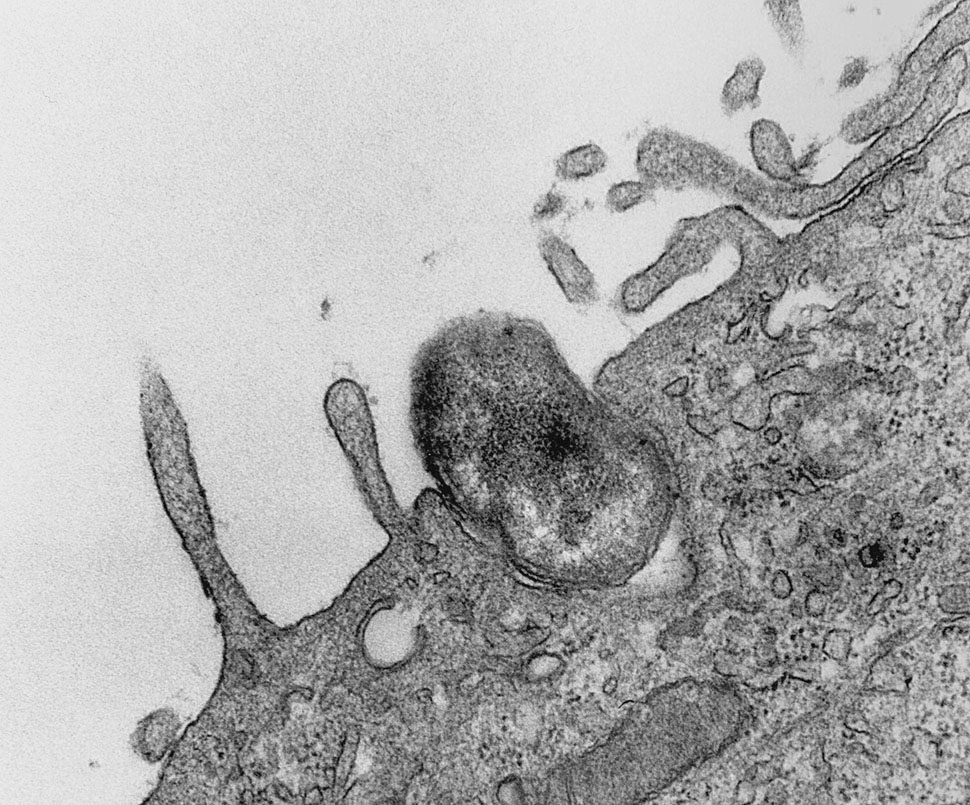
Margulis’s paper was highly speculative, but it provided some clear, testable hypotheses. If mitochondria and chloroplasts were descended from free-living bacteria, then it should be possible to determine from their DNA what those free-living bacteria were. At the time Margulis wrote her paper, chemical analysis of DNA was in its infancy, and she couldn’t use this technique. But by the mid-70s, analyzing DNA had become routine, and it was DNA evidence that clinched her case: By comparing the sequences of mitochondrial and chloroplast DNA to a wide range of bacterial DNA, researchers discovered that mitochondrial DNA closely resembled DNA from a small bacterium called Rikettsia–interestingly, a type of intracellular parasite that burrows inside other cells in order to live. Chloroplast DNA, they discovered, is essentially the same as cyanobacterial DNA.
Cyanobacteria, once mistakenly known as blue-green algae, were the first photosynthesizers on Earth, evolving some 2.5 billion years ago. By taking up residence in a larger host cell, these smaller bacteria endowed the host cell with the capacity to photosynthesize and thus paved the way for the evolution of green plants. “Plants are something that hold up cyanobacteria. That’s all plants are,” Margulis said in a 2006 interview with Astrobiology Magazine. “Fundamentally, if you cut them out of the plant cell, and throw away the rest of the plant cell, the little green dot is the only thing that can do that oxygen production. That is the greatest achievement of life on Earth, and it occurred extremely early in the history of life.”