INFOGRAPHIC 22.2
RADIOACTIVE DECAY
2
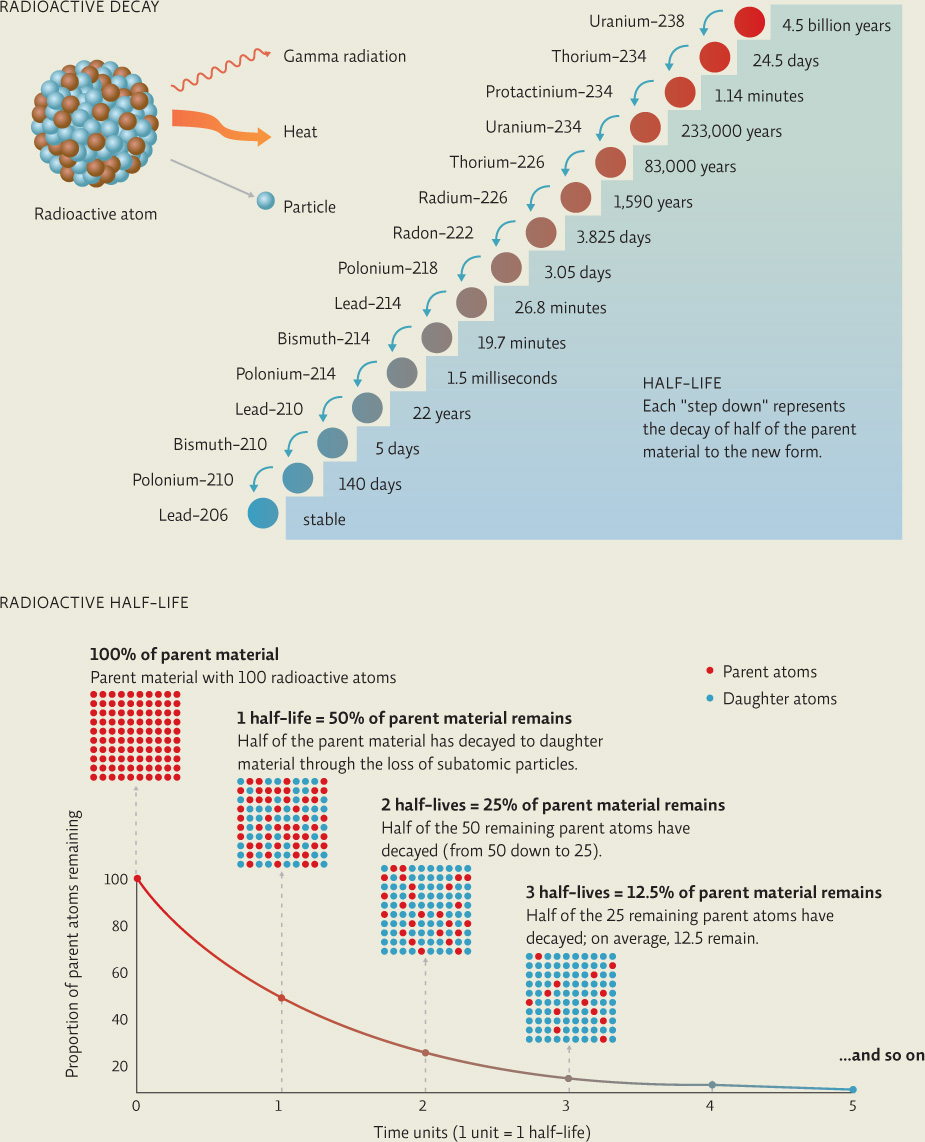
The rate of decay for a given radioactive isotope is predictable and expressed as a half-life—the amount of time it takes for half of the original radioactive material (parent) to decay to the new daughter material (a new isotope, or even a new atom if protons are lost). Radioactive isotopes and their daughter radioactive isotopes continue to decay until they form a stable isotope that no longer loses particles. For instance, U-238 decays initially to thorium-234, which itself will decay over time. The entire decay sequence of U-238 includes progression through at least 13 isotopes (each step with its own half-life that ranges from milliseconds to thousands of years) until the final isotope decays into lead-206, a stable atom.