pH AND OCEAN ACIDIFICATION
The pH scale is a measure of how acidic or basic a solution is. It is a measurement that compares the proportion of hydrogen (H+) or hydroxide (OH−) ions in a solution (ions are charged atoms or molecules). Water (H2O) can dissociate into hydroxide ions (OH−) and hydrogen ions (H+). In pure water, there is always one H+ for every OH−. These solutions are neutral, with a pH of 7.0. Acids have a pH lower than 7.0 and have extra H+ ions in the solution. Bases (alkaline solutions) have a pH higher than 7.0 and have extra OH− ions in the solution.
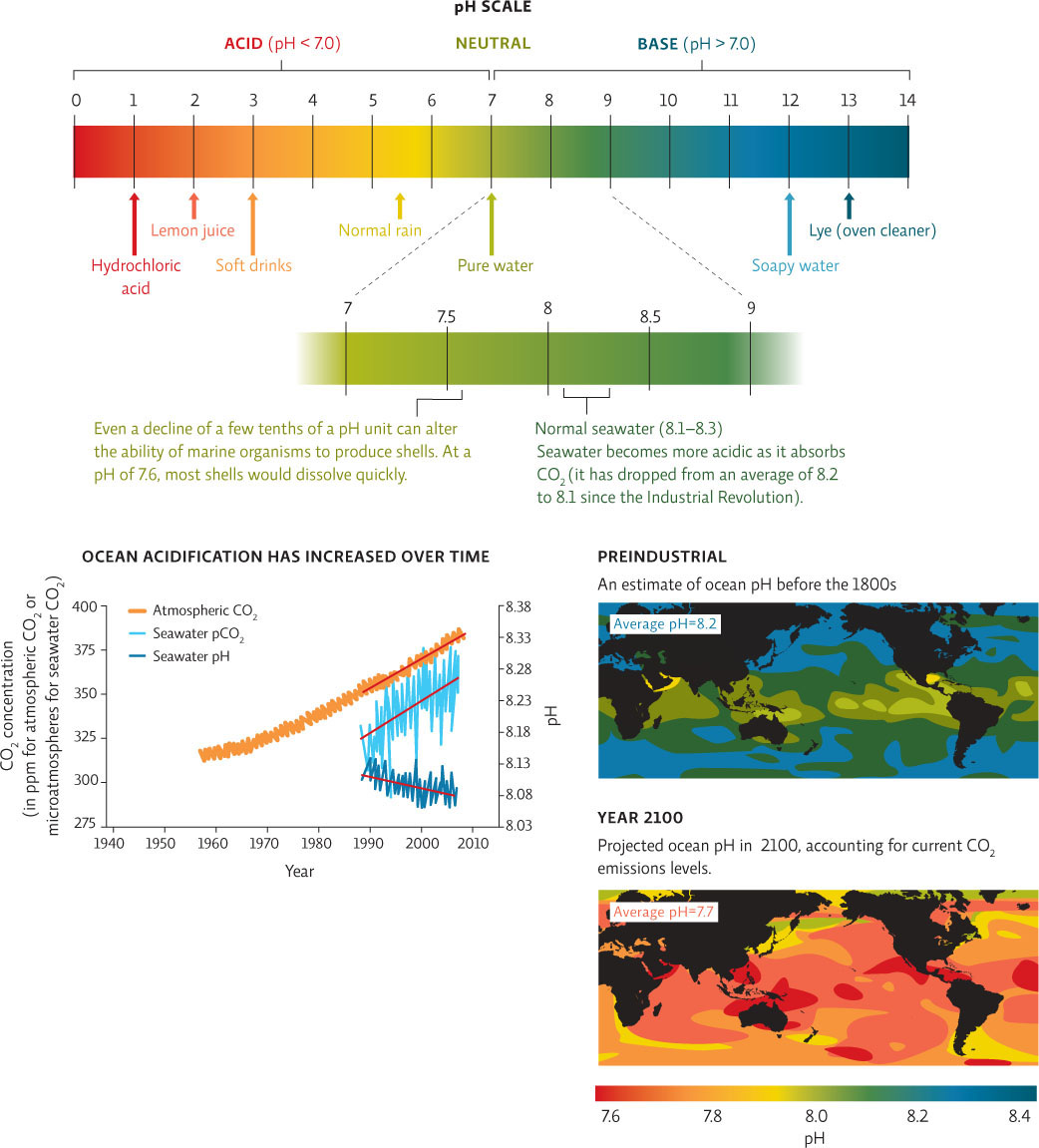
The increase in ocean acidity correlates well with the increases in CO2 dissolved in seawater and atmospheric CO2 concentrations.
Ocean pH will continue to drop significantly if we do not curtail fossil fuel use and our release of extra CO2.