Can coal’s emissions be cleaned up?
Of course it’s not just the mining and processing of coal that pollute the environment.
When coal is burned to produce heat energy, it releases a range of toxic substances that damage the environment and threaten human health: gases (sulfur dioxide, carbon monoxide, nitrogen oxide, and planet-warming carbon dioxide), heavy metals (such as mercury and arsenic), radioactive material (uranium and thorium), and particulate matter (soot) with particles small enough to irritate lung tissue or even enter the bloodstream if inhaled. Until December 2011, there were no regulations to limit how much of these toxic air emissions power plants could release.
According to the UN’s 2013 Global Mercury Assessment, worldwide, coal burning accounts for the release of an estimated 474 metric tons (520 U.S. tons) of mercury into the atmosphere (24% of the total anthropogenic mercury release globally); the EPA estimates that 50% of mercury emissions in the United States are from power plants. Mercury released into the atmosphere can be transported around the world; between 20% and 30% of the mercury found in North America originates in areas as far away as East Asia. Much of this mercury finds its way into the world’s oceans. In fact, the mercury content of the top 100 meters (330 feet) of oceans has doubled over the past 100 years due to anthropogenic emissions. And this extra mercury is finding its way into the organisms (including humans) that consume seafood. The mercury content of ancient and present-day samples of teeth, bone, and hair were analyzed and revealed a dramatic and rapid increase of mercury between preindustrial and modern times; levels in the late 1900s were roughly 10 times greater than levels before the Industrial Revolution. This has ecological consequences but also has direct impacts on human health: The main way humans are exposed to mercury is by eating contaminated fish, especially top predators like tuna that biomagnify mercury (see Chapter 3). The EPA’s new Mercury and Air Toxic Standards will reduce these emissions by 90% and are predicted to save up to $90 billion in human health costs by 2016 (while only costing $9.6 billion to implement).

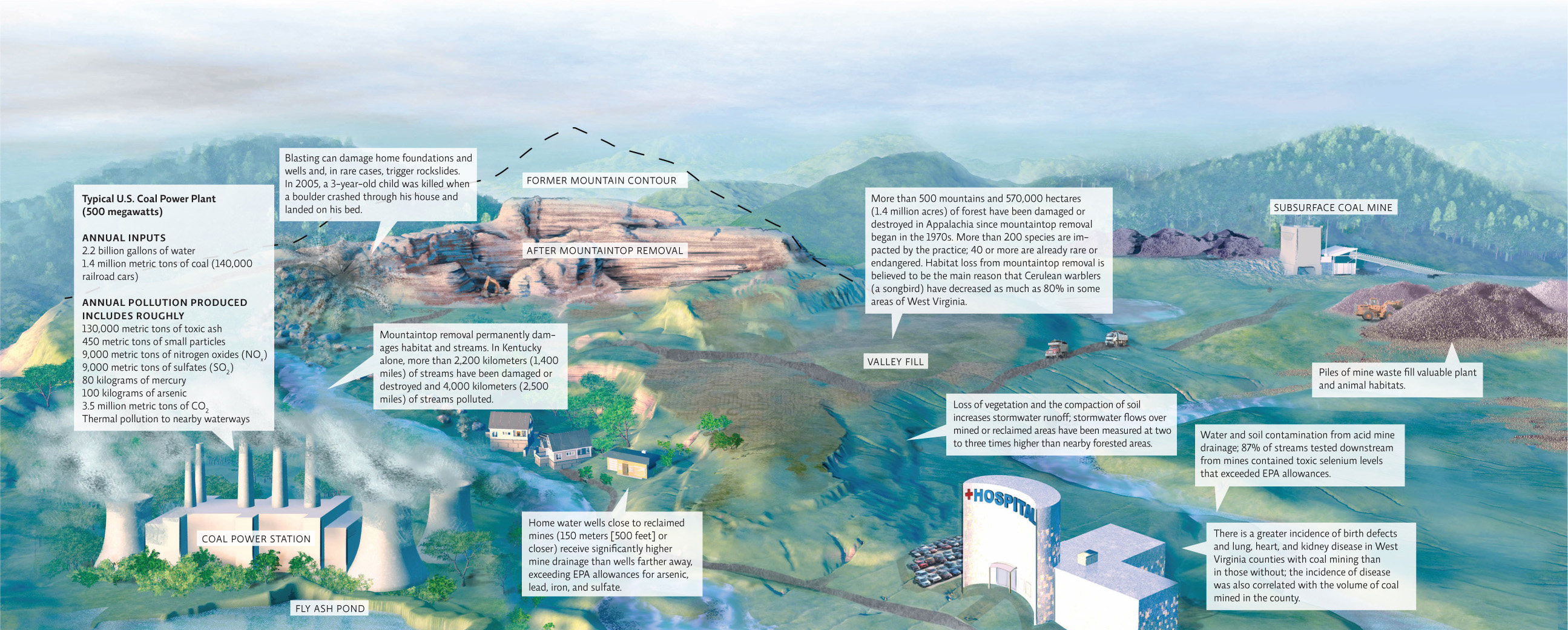
Coal-fired power plants also generate tons of toxic fly ash—fine ashen particles made up mostly of silica. Some of that ash is diverted to industry, where it’s used in concrete production. But the majority of it is buried in hazardous waste landfills or stored in open ponds like the ones used to contain the slurry waste from mining. In 2008, following a heavy rain event, a 15-meter-tall (50-foot-tall) dike from a pond holding coal fly ash from the TVA Kingston Fossil Plant in Tennessee failed, releasing 4.2 billion liters (1.1 billion gallons) of fly ash into nearby rivers and coating vast expanses of riverside land. The EPA estimates that cleanup would cost $1.2 billion—and more when property damage and lawsuit settlements were factored in. Federal regulators have since identified more than 100 other ash ponds at risk for breach. INFOGRAPHIC 18.6
The use of coal contributes to environmental and health problems when it is mined or burned. Surface mining, especially mountaintop removal, produces the most environmental damage. Miners and people living in coal mining communities are most at risk for health problems, but air pollution from burning coal affects individuals and ecosystems far removed from the actual power plant.

Which of the problems associated with extraction and burning of coal concern you the most: the environmental damage or the social costs? Explain.
Answers will vary. Those concerned with environmental damage may mention things like habitat destruction and its effect on species, the loss of ecosystem services, or the loss of forests leading to fewer recreational opportunities. Those concerned with the social aspects may mention things like the problems or declines in property values or community integrity. Some students may be unwilling to choose one over the other, giving both equal weight.
In 2011, public health researchers from Harvard University tallied up the costs of mining and using coal. The analysis took into account many (but not all) of the external costs from mining, shipping, burning, and waste production— that is, the costs that are not currently reflected in the market cost of coal. They estimated that coal costs the American public between $300 and $500 billion a year in externalized costs (health, environmental, and property costs). This amounts to an extra $0.10 to $0.26 per kWh in external costs—in some cases more than twice what consumers actually pay.
So what do we do? On one hand, we rely on coal to power our society. On the other, the processes of mining and then burning it are hurting us and our environment as much as they are sustaining our way of life.
One potential solution is clean coal technology— technology that minimizes the amount of pollution produced by coal. For example, scientists and engineers around the world are working on ways to capture the gases emitted from burning coal. We already have the capacity to capture some emissions like mercury, particulate matter, and sulfur (see Chapter 20).
The next big challenge is carbon capture and sequestration (CCS)—the capture and storage of CO2 in a way that prevents it from reentering the atmosphere. There are many approaches to CCS; some of them promise to capture more than 90% of CO2 emissions from coal-fired power plants. But most are still in the research and development stage, and so far, progress has been hindered by the cost of capturing the carbon and by a dearth of good storage options. The extra energy needed to implement CCS must also be considered: Estimates for the additional energy needed range from 25% to 40%—this means 25—40% more coal that must be mined, processed, transported, and burned. INFOGRAPHIC 18.7
The capture of CO2 and subsequent storage that prevents it from reentering the atmosphere will greatly decrease coal’s contribution to global climate change. A variety of CCS methods are currently in research and development or are being tested as pilot programs.
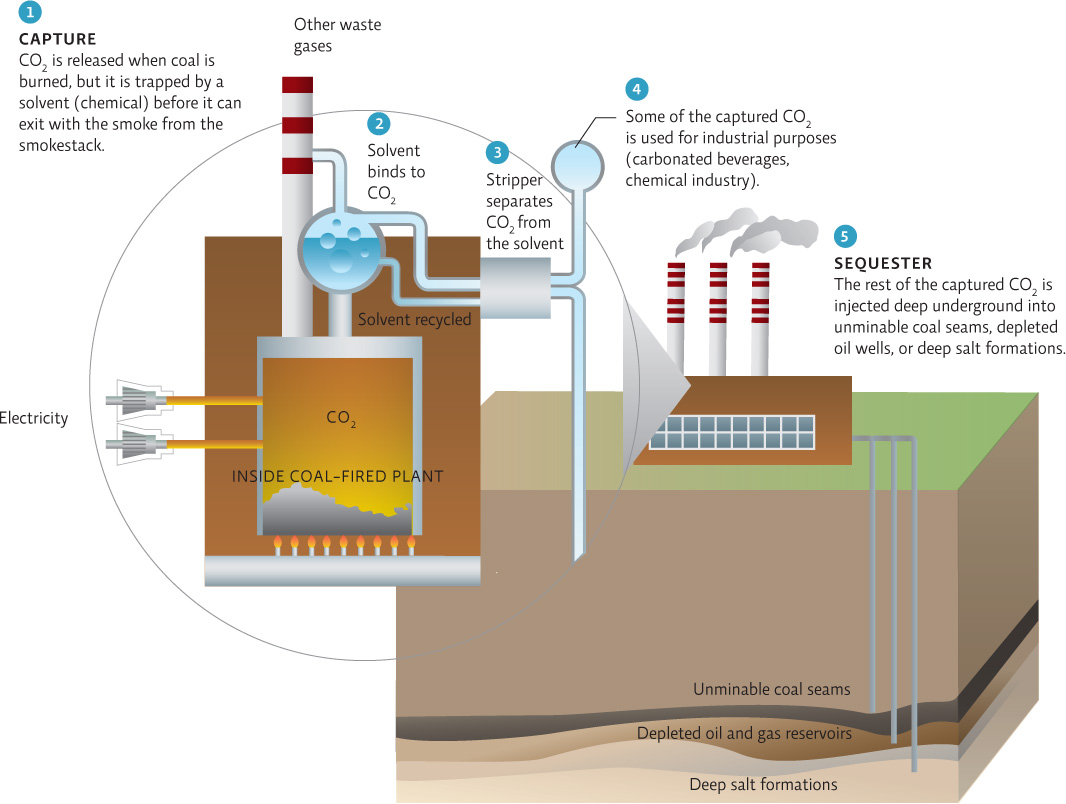

If successful, CCS will reduce coal’s contribution to climate change—a significant outcome. What impact would it have on the negative consequences of extracting coal?
It will not reduce the negative effects of extracting coal -- the coal will still be extracted and burned. It may even increase the need to extract even more coal if the energy needed to fuel the CCS process comes from burning even more coal.
carbon capture and sequestration (CCS)
The process of removing carbon from fuel combustion emissions or other sources and storing it to prevent its release into the atmosphere.
Another emerging clean coal technology involves chemically removing some of coal’s contaminants before burning it. While it still contains some toxic substances, the final product (a liquid or gaseous fuel, depending on the process used), is cleaner than the original coal. Of course, this process requires energy (thus lowering coal’s EROEI) and generates hazardous waste (from the toxic substances found in the coal) that still has to be dealt with.
KEY CONCEPT 18.7
The negative impact of burning coal can be addressed by capturing pollutants before they are released or converting coal to a liquid or gaseous fuel that burns cleaner.
In fact, as critics are quick to point out, coal can never be truly clean. Each of these “clean coal” technologies produces its own toxic by-products, and none of them eliminate the need to mine coal from deep within Earth’s surface.
Even if we can’t easily avoid using coal or make it totally clean, we can certainly try to use less of it. One way to accomplish this is to design and use more energy-efficient appliances. Less than 40% of the energy from coal is converted to electricity; in addition, the amount of that energy that goes toward powering appliances depends on how efficient those appliances are. We can also reduce our coal consumption through simple conservation efforts like turning off lights and electronics when we’re not using them. Every kilowatt-hour of electricity conserved means roughly 0.5 kilograms (1 pound) less of coal being burned. And a growing number of public utilities now offer “green power” programs in which users can choose to buy electricity that has been generated by solar or wind power instead of coal. (For more on energy efficiency and conservation, see Chapter 23.)