The international community got together to meet the problem head on.
Even while scientists were still trying to understand why the ozone layer was depleting, they knew they had to do something about it. Concerns raised by researchers in the 1970s had already led to a ban on CFCs for use as a propellant in hairsprays and other products in some countries, including the United States.
In 1985, a group of experts from around the world met in Vienna, Austria, to discuss ways to research and solve the problem. This set the stage for the international community to come together in Montreal, Canada, in 1987, to produce a plan to deal with the problem of ozone depletion. The plan, called the Montreal Protocol, would involve sacrifices—notably, phasing out dangerous chemicals including CFCs. At that first meeting in September 1987, only two dozen governments signed on to the protocol.
Montreal Protocol
An international treaty that laid out plans to phase out ozone-depleting chemicals such as CFCs.
As the evidence mounted, industry, the public, and governments began to realize the seriousness of the ozone problem. By 2009, the protocol was eventually ratified by all 196 countries in the world when East Timor signed on.
The Montreal Protocol, administered by the United Nations, outlined a series of deadlines over the next decade for cutting back production of CFCs. Governments would have to put in place their own plans for achieving a desired outcome, or policy, for reducing CFCs.
policy
A formalized plan that addresses a desired outcome or goal.
Policy has been described as translating our values into action. Science provides information we can use to make informed decisions to protect our health, safety, or environment. Society then decides what “ought” to be addressed (Do we address ozone depletion?) and hopefully uses the best scientific information available to set reasonable policies that take into account the ecological, economic, social, and political issues at stake.
KEY CONCEPT 2.8
Policy makers should base decisions on the best scientific evidence available, and they should employ the precautionary principle and adaptive management when appropriate.
To achieve the goals of the Montreal Protocol, the U.S. government required that CFCs be phased out starting in the early 1990s, through regulations from the U.S. Environmental Protection Agency, the federal agency responsible for seeing that environmental laws are followed.
Interestingly, the 1987 Montreal Protocol and the international commitment to address CFCs and ozone depletion came before Susan Solomon’s definitive studies were published in 1988. This is an example of applying the precautionary principle—acting in the face of uncertainty when there is a chance that serious consequences might occur. By 1987, though we didn’t know all the details, we knew enough to take action. As more information poured in, it quickly became apparent that the Montreal Protocol targets would not be sufficient to stop ozone depletion. Amendments to the protocol are still proposed and negotiated in annual meetings that strengthen the response and adjust the target dates to phase out harmful compounds. This is an ongoing process, and it is an example of adaptive management—allowing room for altering strategies as new information comes in or the situation itself changes. INFOGRAPHIC 2.7
precautionary principle
A principle that encourages acting in a way that leaves a margin of safety when there is a potential for serious harm but uncertainty about the form or magnitude of that harm.
adaptive management
A plan that allows room for altering strategies as new information becomes available or as the situation itself changes.
Actual and projected change over time for total global emissions of ozone-depleting substances (ODS) with and without the Montreal Protocol and its amendments. Adjustments to the phase-out schedule of various ODSs in the form of amendments represent the success of adaptive management in dealing with complex environmental issues.
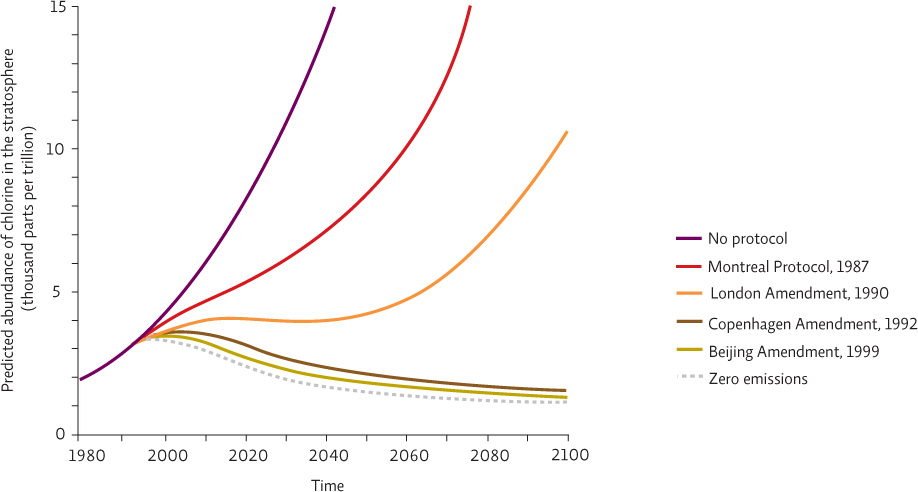

What type of new information might have led policy makers to amend the Montreal Protocol?
New ozone depleting substances were identified and added to the protocol. Researchers could have also updated the estimates for the ozone depleting effects of various ODS (their ability to contribute Cl to the atmosphere) requiring changes to reach the desired outcome.
This same approach is needed to address other global environmental issues, such as climate change. We cannot know the exact details of future climate change impacts before they occur, but we do have a good idea of what we are facing. By acting now, we can lessen the severity of those impacts and adjust our response as we go along.
Fortunately, companies that produced CFCs, such as DuPont, were already researching alternative chemicals in the lab that could serve the same purposes with less damage. If companies could sell a CFC alternative just as easily, then cutting back on CFCs would not hurt them financially. Still, some of the replacements, particularly some hydrochlorofluorocarbons (HCFCs) used in refrigerants, would eventually also prove to be detrimental to ozone. In addition, illegal stockpiles and old refrigerators continue to release some CFCs, though that amount is decreasing rapidly, thanks to the Montreal Protocol. Pockets of CFCs remain because of continued legal “essential” uses, such as for medicines and nuclear power.
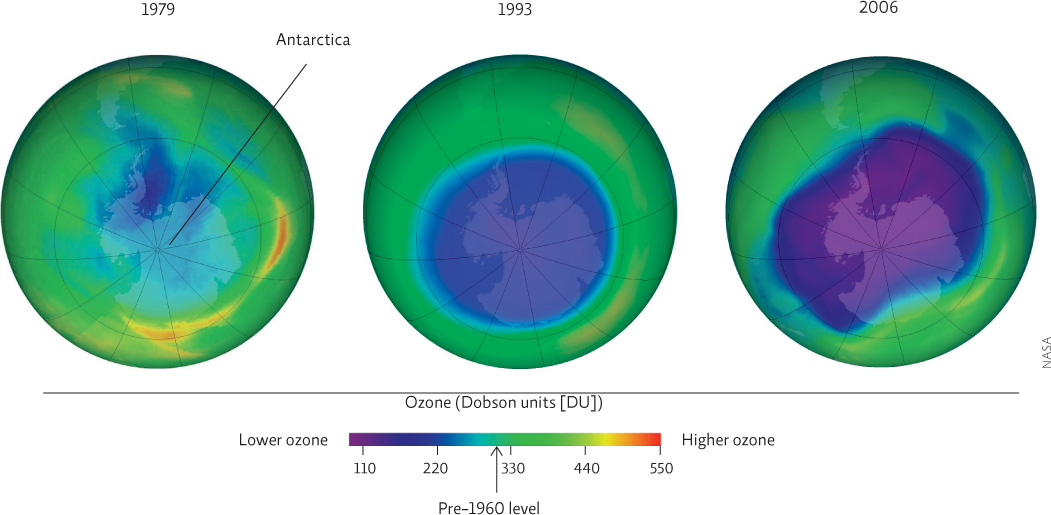
Today, ozone-depleting compounds in the stratosphere are decreasing at a rate consistent with the policy changes of the Montreal Protocol and all its subsequent amendments. Outside polar regions, ozone levels declined through the 1990s but seem to be holding steady more recently. Still, the Montreal Protocol has been hailed as the most successful international environmental agreement in history.
There is still much we don’t understand about the atmospheric chemistry of ozone depletion. Just when it seemed that ozone was beginning to recover, the 2004–2005 Arctic winter was unusually cold, and ozone loss was very high that year. This was eclipsed by a record 40% loss of ozone over the Arctic in spring of 2011. And Antarctica set two new records for ozone depletion in 2006—the widest hole ever observed and the deepest hole ever observed, with some areas near 90% depletion. This tells us that even though CFCs are declining—the 2012 Antarctic hole was smaller than it had been in 21 years, thanks in part to a warmer-than-average Antarctic stratosphere—very cold winters will still give us years of higher-than-expected ozone depletion.
Projections estimate that midlatitude areas should be back to pre-1980 ozone levels by 2050, and polar regions should be back by 2075; however, the next 15 years should show periodic large declines and produce large “ozone holes” in very cold years like those seen in 2006 and 2011. Although she retired from NOAA in 2011, Solomon continues her research on CFCs and is both hopeful and realistic. “It’s clear that ozone will ultimately recover, but it’s also clear that it will take many decades to do so,” she says. “It has been a real privilege to work on such an interesting problem and to feel that the world found it useful in making choices about the Montreal Protocol.”
By acting now, we can lessen the severity of those impacts and adjust our response as we go along.
Select References:
Abarca, J. F., & C. C. Casiccia. (2002). Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: Southern Chile. Photodermatology, Photoimmunology & Photomedicine, 18(6): 294–302.
Farman, J., et al. (1985). Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature, 315(6016): 207–210.
Menzies, S. W., et al. (1991). Ultraviolet radiation-induced murine tumors produced in the absence of ultraviolet radiation-induced systemic tumor immunosuppression. Cancer Research, 51(11): 2773–2779.
Molina, M. J., & F. S. Rowland. (1974). Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature, 249(5460): 810–812.
NASA. “Ozone Hole Watch,” http://ozonewatch.gsfc.nasa.gov.
Solomon, S., et al. (1987). Visible spectroscopy at McMurdo station, Antarctica: 2. Observations of OClO, Journal of Geophysical Research, 92(D7): 8329–8338.
Solomon, S., et al. (1988). Observation of the nighttime abundance of OClO in the winter stratosphere above Thule, Greenland. Science, 242(4878): 550–555.
PERSONAL CHOICES THAT HELP
The depletion of the ozone layer is a great example of how science documented a problem and its cause, and public action confronted the problem. All the environmental changes we face, from rising levels of greenhouse gases to loss of biodiversity, can be addressed using science. How scientific information is or is not put into action has far-reaching consequences, making science literacy a matter of importance for every individual.
Individual Steps
•Practice thinking like a scientist. Go outside for 10 minutes and observe the world around you. Make observations of what you see or hear. What predictions could you make from your observations? How could you test them?
•Stay informed. Read or watch a science-related article or show once a month.
Group Action
•Demonstrate the importance of scientific literacy to your friends and family. Develop three additional questions from the material in the chapter and discuss them over dinner.
•Support science education: Find out about public lectures and programs in your area and attend one with your friends.
Policy Change
•Attend a city council or county board meeting to see how policy issues are addressed in your area.
•Make knowledgeable voting decisions on ballot initiatives.
•Serve on local civic committees that address environmental issues in your community.
