16.3 Nutrient enrichment can lead to oxygen depletion.
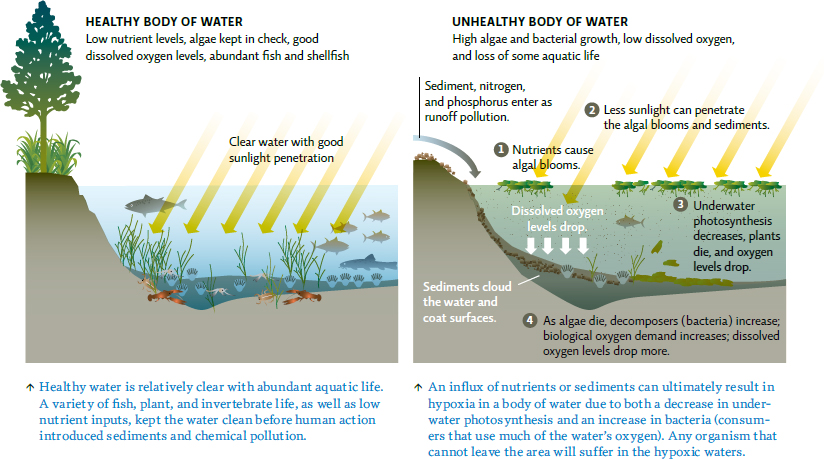
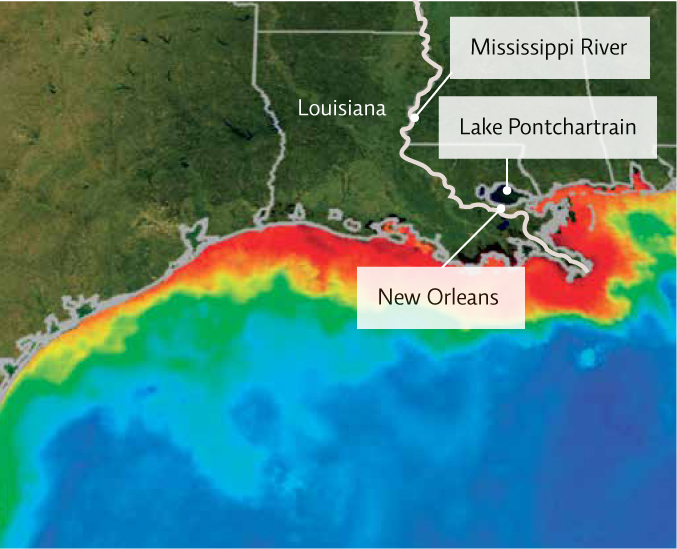
The huge influx of nutrients that run off from farms, golf courses, and lawns into Lake Erie and the rivers and streams that feed into it (tributaries) is the first step in a process known as eutrophication, or more specifically cultural eutrophication (nutrient enrichment caused by human activities). Because nutrients—primarily phosphorus but also nitrogen—fuel plant growth, excess amounts trigger explosions of algae. In the 1970s, Lake Erie wasn’t blue—it was a pea-green colour, full of algae. While not necessarily toxic, pollution that adds nutrients to a body of water can throw its natural ecosystem out of balance, spurring the growth of some organisms and reordering the entire ecological community. “The Great Lakes have aged over many thousands of years,” explains Watson. “They developed a balanced system that functioned well, and enabled them to survive dramatic shifts in climate and other changes. Within 200 years, humans have completely altered the entire area around the Great Lakes so rapidly, they never had a chance to recover.”
Algae aren’t just a cosmetic problem—blooms may also contain one or more species of cyanobacteria (formerly called blue-green algae, because scientists initially thought they were algae), a type of photosynthetic bacteria. Some cyanobacteria produce potent toxins that can harm the liver and even cause death after chronic exposure. In Brazil, 46 people receiving dialysis died after the facility unknowingly used water that contained cyanobacterial toxins. In Canada, most people won’t accidentally drink contaminated water because of the foul smell produced by cyanobacteria, but pets and livestock are less discriminating. Children are also more vulnerable due to their smaller size; even small amounts can make them sick.
But not every bloom produces toxins, and even when toxins are present, they are not always the source of the biggest problems. Though algae release some oxygen into the water as a by-product of photosynthesis, thick layers of surface algae also block sunlight from reaching underwater plants, causing them to die. As a result, levels of dissolved oxygen (DO) in the water plummet—a condition known as hypoxia. Water can only hold a limited amount of oxygen, much less than that found in air, so even a small decrease can have immediate effects on other aquatic life.
An influx of nutrients or an algal bloom also cause an explosion of bacterial decomposers that consume these nutrients and dead algae; as part of this process, bacteria consume even more oxygen, which reduces DO levels even further. When people referred to Lake Erie as “dead” decades ago, they weren’t so far off—hypoxic areas are often referred to as dead zones. “But in fact, ‘dead zones’ are not ‘dead’,” says Watson—“they are full of bacteria.” [infographic 16.2]
285