3.2 The Electrochemical Actions of Neurons: Information Processing
Our thoughts, feelings, and actions depend on neural communication, but how does it happen? The communication of information within and between neurons proceeds in two stages:
Conduction is the movement of an electric signal within a neuron, from the dendrites to the cell body, then throughout the axon.
Transmission is movement of electric signals from one neuron to another over the synapse.
Together, these stages are what scientists generally refer to as the electrochemical action of neurons.
3.2.1 Electric Signalling: Conducting Information within a Neuron
The neuron’s cell membrane has small pores that act as channels to allow small electrically charged molecules, called ions, to flow in and out of the cell. It is this flow of ions across the neuron’s cell membrane that creates the conduction of an electric signal within the neuron. How does it happen?
3.2.1.1 The Resting Potential: The Origin of the Neuron’s Electrical Properties

Neurons have a natural electric charge called the resting potential, the difference in electric charge when the neuron is at rest (Kandel, 2000). When first discovered by biologists in the 1930s, the resting potential was measured at about about −70 mV. This is a much smaller charge than a typical battery, for example a 9-
The resting potential arises from the difference in concentrations of ions inside and outside the neuron’s cell membrane (see FIGURE 3.5a). Ions can carry a positive (+) or a negative (−) charge. In the resting state, there is a high concentration of a positively charged ion, potassium (K+), as well as negatively charged protein ions (A−), inside the neuron’s cell membrane compared to outside it. By contrast, there is a high concentration of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl−) outside the neuron’s cell membrane.
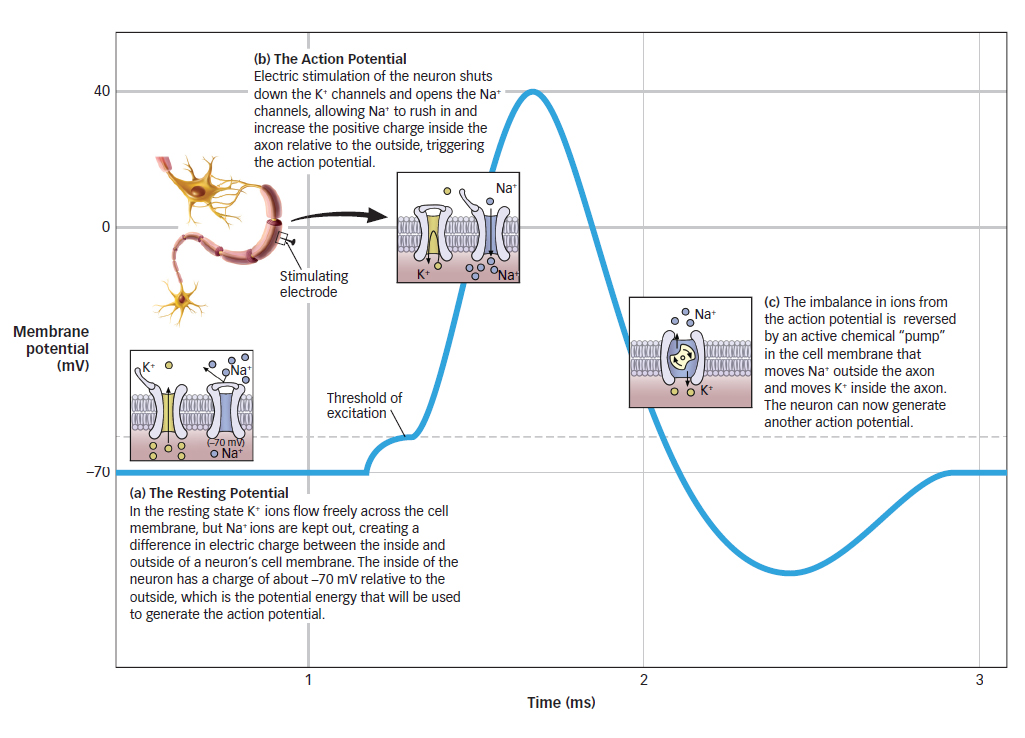
 Figure 3.5: The Resting and Action Potentials Neurons have a natural electric charge called a resting potential. Electric stimulation causes an action potential.
Figure 3.5: The Resting and Action Potentials Neurons have a natural electric charge called a resting potential. Electric stimulation causes an action potential.
What difference between the inside and outside of the neuron’s cell membrane creates the resting potential?
The concentration of K+ inside and outside a neuron is controlled by channels in the cell membrane that allow K+ ions to flow in and out of the neuron. In the resting state, the channels that allow K+ ions to flow freely across the cell membrane are open, while channels that allow the flow of Na+ (and A−, and Cl−) are generally closed. Because of the naturally higher concentration of K+ ions inside the neuron, some K+ molecules move out of the neuron through the open channels, leaving the inside of the neuron with a charge of about −70 mV relative to the outside. Like a dam that holds back a river until the floodgates are released, resting potential is potential energy, because it creates the environment for a possible electrical impulse.
3.2.1.2 The Action Potential: Sending Signals across the Neuron
The neuron maintains its resting potential most of the time. However, the biologists working with the giant squid axon noticed that when they stimulated the axon with a brief electric shock, it resulted in the conduction of an electric impulse down the length of the axon producing a signal (Hausser, 2000; Hodgkin & Huxley, 1939). This electric impulse is called an action potential, an electric signal that is conducted along the length of a neuron’s axon to a synapse.
Why is an action potential an all-
The action potential occurred only when the electric shock reached a certain level, or threshold. When the shock was below this threshold, the researchers recorded only tiny signals, which dissipated rapidly. When the shock reached the threshold, a much larger signal, the action potential, was observed. Interestingly, increases in the electric shock above the threshold did not increase the strength of the action potential. The action potential is all or none: Electric stimulation below the threshold fails to produce an action potential, whereas electric stimulation at or above the threshold always produces the action potential. The action potential always occurs with exactly the same characteristics and at the same magnitude regardless of whether the stimulus is at or above the threshold.

The biologists working with the giant squid axon observed another surprising property of the action potential: They measured a peak charge of about +40 mV, which is well above zero. This suggests that the mechanism driving the action potential could not simply be the loss of the −70 mV resting potential because this would have only brought the charge back to zero. So why does the action potential reach a value above zero?
The action potential occurs when there is a change in the state of the axon’s membrane channels. Remember, during the resting potential, the channels that allow K+ to flow out are open. However, when an electric charge is raised to the threshold value, these channels briefly shut down, and channels that allow the flow of positively charged sodium ions (Na+) are opened (see FIGURE 3.5b). We have seen already that Na+ is typically much more concentrated outside the axon than inside. When the channels open, those positively charged ions (Na+) flow inside, increasing the positive charge inside the axon relative to that outside. This flow of Na+ into the axon pushes the action potential to its maximum value of +40 mV.
After the action potential reaches its maximum, the membrane channels return to their original state, and K+ flows out until the axon returns to its resting potential. This leaves a lot of extra Na+ ions inside the axon and a lot of extra K+ ions outside the axon. During this period when the ions are imbalanced, the neuron cannot initiate another action potential, so it is said to be in a refractory period, the time following an action potential during which a new action potential cannot be initiated. The imbalance in ions is eventually reversed by an active chemical “pump” in the cell membrane that moves Na+ outside the axon and moves K+ inside the axon (the pump does not operate during the action potential; see FIGURE 3.5c).
So far we have described how the action potential occurs at one point in the neuron. But how does this electric charge move down the axon? It is a domino effect. When an action potential is generated at the beginning of the axon, it spreads a short distance, which generates an action potential at a nearby location on the axon. That action potential also spreads, initiating an action potential at another nearby location, and so on, thus conducting the charge down the length of the axon. This simple mechanism ensures that the action potential travels the full length of the axon and that it maintains its full intensity at each step, regardless of the distance travelled.
The myelin sheath facilitates the conduction of the action potential. Myelin does not cover the entire axon; rather, it clumps around the axon with little break points between clumps, looking kind of like sausage links. These breakpoints are called the nodes of Ranvier, after French pathologist Louis-
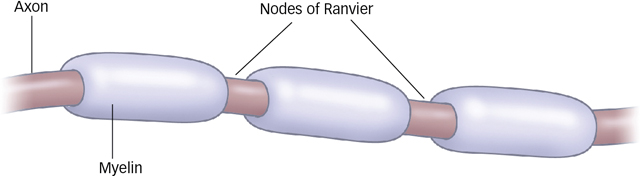
 Figure 3.6: Myelin and Nodes of Ranvier Myelin is formed by a type of glial cell, and it wraps around a neuron’s axon to speed the movement of the action potential along the length of the axon. Breaks in the myelin sheath are called the nodes of Ranvier. The electric impulse appears to jump from node to node, thereby speeding the conduction of information down the axon.
Figure 3.6: Myelin and Nodes of Ranvier Myelin is formed by a type of glial cell, and it wraps around a neuron’s axon to speed the movement of the action potential along the length of the axon. Breaks in the myelin sheath are called the nodes of Ranvier. The electric impulse appears to jump from node to node, thereby speeding the conduction of information down the axon.
3.2.2 Chemical Signalling: Transmission between Neurons
When the action potential reaches the end of an axon, you might think that it stops there. After all, the synaptic space between neurons means that the axon of one neuron and the neighbouring neuron’s dendrites do not actually touch one another. However, the electric charge of the action potential triggers a chemical signal that can cross the relatively small space of the synaptic cleft.
How does a neuron communicate with another neuron?
Axons usually end in terminal buttons, knoblike structures that branch out from an axon. A terminal button is filled with tiny vesicles, or “bags,” that contain neurotransmitters, chemicals that transmit information across the synapse to a receiving neuron’s dendrites. The dendrites of the receiving neuron contain receptors, parts of the cell membrane that receive neurotransmitters and either initiate or prevent a new electric signal.
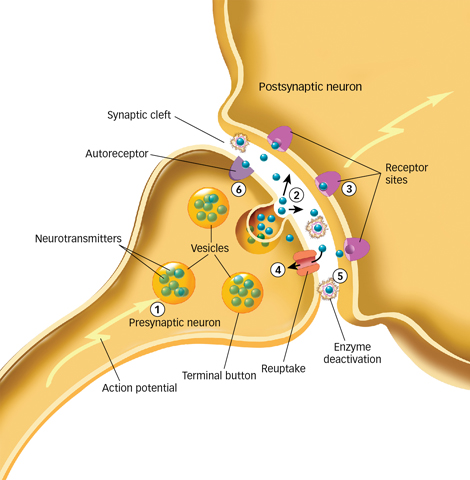
 Figure 3.7: Synaptic Transmission (1) The action potential travels down the axon and (2) stimulates the release of neurotransmitters from vesicles. (3) The neurotransmitters are released into the synapse, where they float over to bind with receptor sites on a dendrite of a postsynaptic neuron, initiating a new action potential. The neurotransmitters are cleared out of the synapse by (4) reuptake into the sending neuron, (5) being broken down by enzymes in the synapse, or (6) binding to autoreceptors on the sending neuron.
Figure 3.7: Synaptic Transmission (1) The action potential travels down the axon and (2) stimulates the release of neurotransmitters from vesicles. (3) The neurotransmitters are released into the synapse, where they float over to bind with receptor sites on a dendrite of a postsynaptic neuron, initiating a new action potential. The neurotransmitters are cleared out of the synapse by (4) reuptake into the sending neuron, (5) being broken down by enzymes in the synapse, or (6) binding to autoreceptors on the sending neuron.
As K+ and Na+ flow across a cell membrane, they move the sending neuron, or presynaptic neuron, from a resting potential to an action potential. The action potential travels down the length of the axon to the terminal buttons, where it stimulates the release of neurotransmitters from vesicles into the synapse. These neurotransmitters float across the synapse and bind to receptor sites on a nearby dendrite of the receiving neuron, or postsynaptic neuron. A new action potential is initiated in that neuron, and the process continues down that neuron’s axon to the next synapse and the next neuron. This electrochemical action, called synaptic transmission (see FIGURE 3.7), allows neurons to communicate with one another and ultimately underlies your thoughts, emotions, and behaviour.
Now that you understand the basic process of how information moves from one neuron to another, we can refine things a bit. You will recall that a given neuron may make a few thousand synaptic connections with other neurons, so what tells the dendrites which of the neurotransmitters flooding into the synapse to receive? One answer is that neurons tend to form pathways in the brain that are characterized by specific types of neurotransmitters; one neurotransmitter might be prevalent in one part of the brain, whereas a different neurotransmitter might be prevalent in a different part of the brain.
A second answer is that neurotransmitters and receptor sites act like a lock-
So what happens to the neurotransmitters left in the synapse after the chemical message is relayed to the postsynaptic neuron? Something must make neurotransmitters stop acting on neurons; otherwise, there would be no end to the signals that they send. Neurotransmitters leave the synapse through three processes (see Figure 3.7). First, reuptake occurs when neurotransmitters are reabsorbed by the terminal buttons of the presynaptic neuron’s axon. Second, neurotransmitters can be destroyed by enzymes in the synapse in a process called enzyme deactivation; specific enzymes break down specific neurotransmitters. Finally, neurotransmitters can bind to the receptor sites called autoreceptors on the presynaptic neurons. Autoreceptors detect how much of a neurotransmitter has been released into a synapse and signal the neuron to stop releasing the neurotransmitter when an excess is present.
3.2.2.1 Types and Functions of Neurotransmitters
Given that different kinds of neurotransmitters can activate different kinds of receptors, like a lock and key, you might wonder how many types of neurotransmitters are floating across synapses in your brain right now. Today we know that some 60 chemicals play a role in transmitting information throughout the brain and body, and differentially affect thought, feeling, and behaviour, but a few major classes seem particularly important. We will summarize those here, and we will discuss some of these neurotransmitters again in later chapters.
Acetylcholine (ACh) is a neurotransmitter involved in a number of functions, including voluntary motor control. Acetylcholine is found in neurons of the brain and in the synapses where axons connect to muscles and body organs, such as the heart. Acetylcholine activates muscles to initiate motor behaviour, but it also contributes to the regulation of attention, learning, sleeping, dreaming, and memory (Gais & Born, 2004; Hasselmo, 2006; Wrenn et al., 2006). Alzheimer’s disease, a medical condition involving severe memory impairments (Salmon & Bondi, 2009), is associated with the deterioration of ACh-
producing neurons. Dopamine is a neurotransmitter that regulates motor behaviour, motivation, pleasure, and emotional arousal. Because of its role in basic motivated behaviours, such as seeking pleasure or associating actions with rewards, dopamine plays a role in drug addiction (Baler & Volkow, 2006). High levels of dopamine have been linked to schizophrenia (Winterer & Weinberger, 2004), whereas low levels have been linked to Parkinson’s disease.
Glutamate is the major excitatory neurotransmitter in the brain, meaning that it enhances the transmission of information between neurons. GABA (gamma-aminobutyric acid), in contrast, is the primary inhibitory neurotransmitter in the brain, meaning that it tends to stop the firing of neurons. Too much glutamate, or too little GABA, can cause neurons to become overactive, causing seizures.
Two related neurotransmitters influence mood and arousal: norepinephrine and serotonin. Norepinephrine is particularly involved in states of vigilance, or a heightened awareness of dangers in the environment (Ressler & Nemeroff, 1999). Serotonin is involved in the regulation of sleep and wakefulness, eating, and aggressive behaviour (Dayan & Huys, 2009; Kroeze & Roth, 1998). Because both neurotransmitters affect mood and arousal, low levels of each have been associated with mood disorders (Tamminga et al., 2002).
Page 89 Sandra Wallenhorst of Germany began a 180-
Sandra Wallenhorst of Germany began a 180-km bicycle ride, just one part of the 2009 Ironman World Championship in Hawaii. When athletes such as Wallenhorst engage in extreme sports, they may experience subjective highs that result from the release of endorphins— chemical messengers acting in emotion and pain centres that elevate mood and dull the experience of pain. AP PHOTO/CHRIS STEWARTHow do neurotransmitters create the feeling of runner’s high?
Endorphins are chemicals that act within the pain pathways and emotion centres of the brain (Keefe et al., 2001). The term endorphin is a contraction of endogenous morphine, and that is a pretty apt description. Morphine is a synthetic drug that has a calming and pleasurable effect; an endorphin is an internally produced substance that has similar properties, such as dulling the experience of pain and elevating moods. The “runner’s high” experienced by many athletes as they push their bodies to painful limits of endurance can be explained by the release of endorphins in the brain (Boecker et al., 2008).
Each of these neurotransmitters affects thought, feeling, and behaviour in different ways, so normal functioning involves a delicate balance of each. Even a slight imbalance—
3.2.2.2 How Drugs Mimic Neurotransmitters
Many drugs that affect the nervous system operate by increasing, interfering with, or mimicking the manufacture or function of neurotransmitters (Cooper, Bloom, & Roth, 2003; Sarter, 2006). Agonists are drugs that increase the action of a neurotransmitter. Antagonists are drugs that block the function of a neurotransmitter. Some drugs alter a step in the production or release of the neurotransmitter, whereas others have a chemical structure so similar to a neurotransmitter that the drug is able to bind to that neuron’s receptor. If, by binding to a receptor, a drug activates the neurotransmitter, it is an agonist; if it blocks the action of the neurotransmitter, it is an antagonist (see FIGURE 3.8).
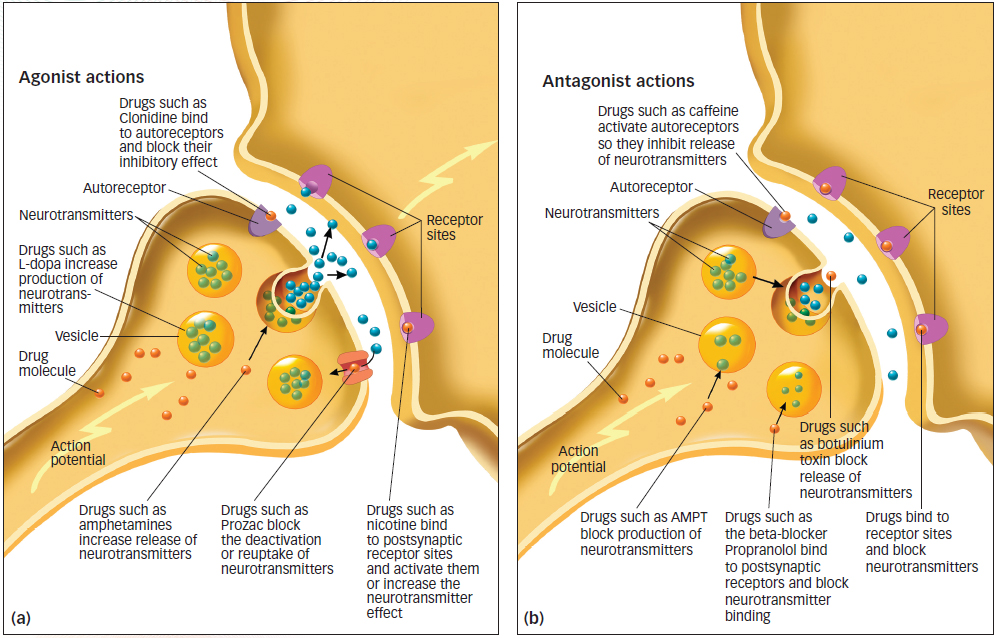
 Figure 3.8: The Actions of Agonist and Antagonist Drugs Agonist and antagonist drugs can enhance or interfere with synaptic transmission at every point in the process: in the production of neurotransmitters, in the release of neurotransmitters, at the autoreceptors, in reuptake, in the postsynaptic receptors, and in the synapse itself.
Figure 3.8: The Actions of Agonist and Antagonist Drugs Agonist and antagonist drugs can enhance or interfere with synaptic transmission at every point in the process: in the production of neurotransmitters, in the release of neurotransmitters, at the autoreceptors, in reuptake, in the postsynaptic receptors, and in the synapse itself.
How does L-
For example, the drug L-

Grasping the toothpaste is nothing compared to the effort it takes to coordinate the two-
Many other drugs, including some street drugs, alter the actions of neurotransmitters. Let us look at a few more examples.
Amphetamine is a popular drug that stimulates the release of norepinephrine and dopamine. In addition, both amphetamine and cocaine prevent the reuptake of norepinephrine, serotonin, and dopamine. The combination of increased release of norepinephrine, serotonin, and dopamine and prevention of their reuptake floods the synapse with those neurotransmitters, resulting in increased activation of their receptors. Both of these drugs therefore are strong agonists, although the psychological effects of the two drugs differ somewhat because of subtle distinctions in where and how they act on the brain. Norepinephrine and dopamine play a critical role in mood control, such that increases in either neurotransmitter result in euphoria, wakefulness, and a burst of energy. However, norepinephrine also increases heart rate. An overdose of amphetamine or cocaine can cause the heart to contract so rapidly that heartbeats do not last long enough to pump blood effectively, leading to fainting and sometimes death.
Prozac, a drug commonly used to treat depression, is another example of a neurotransmitter agonist. Prozac blocks the reuptake of the neurotransmitter serotonin, making it part of a category of drugs called selective serotonin reuptake inhibitors, or SSRIs (Wong, Bymaster, & Engleman, 1995). By blocking reuptake, more of the neurotransmitter remains in the synapse longer and produces greater activation of serotonin receptors. Serotonin elevates mood, which can help relieve depression (Mann, 2005).
An antagonist with important medical implications is a drug called propranolol, one of a class of drugs called beta blockers that obstruct a receptor site for norepinephrine in the heart. Because norepinephrine cannot bind to these receptors when propranolol is present, heart rate slows down, which is helpful for disorders in which the heart beats too fast or irregularly. Beta blockers are also prescribed to reduce the agitation, racing heart, and nervousness associated with stage fright (Mills & Dimsdale, 1991). (For additional discussion of antianxiety and antidepression drug treatments, see the Treatment of Psychological Disorders chapter.)
The conduction of an electric signal within a neuron happens when the resting potential is reversed by an electric impulse called an action potential. An action potential is generated in a neuron when that neuron’s membrane has been sufficiently depolarized. The action potential acts like a signal, travelling down the axon of the cell in which it was generated, triggering the release of neurotransmitters so that the signal is communicated to postsynaptic neurons.
The neuron’s resting potential is due to a difference in electric charge across the neuron’s cell membrane. This charge difference arises because in the resting state, positively charged potassium ions (K+) can move freely across the membrane, whereas positively charged sodium ions (Na+) are kept out of the cell.
If electric signals reach a threshold, this initiates an action potential, an all-
or- none signal that moves down the entire length of the axon. The action potential occurs when K+ channels in the axon membrane close and Na+ channels open, allowing the Na+ ions to flow inside the axon. After the action potential has reached its maximum, a chemical pump reverses the imbalance in ions, returning the neuron to its resting potential. For a brief refractory period, the action potential cannot be re- initiated. Once it is initiated, the action potential spreads down the axon, appearing to jump across the nodes of Ranvier to the synapse. Communication between neurons takes place through synaptic transmission, where an action potential triggers release of neurotransmitters from the terminal buttons of the presynaptic (sending) neuron’s axon, which travel across the synapse to bind with receptors in the postsynaptic (receiving) neuron’s dendrite.
Neurotransmitters bind to dendrites on specific receptor sites. Neurotransmitters leave the synapse through reuptake, through enzyme deactivation, and by binding to autoreceptors.
Some of the major neurotransmitters are acetylcholine (ACh), dopamine, glutamate, GABA, norepinephrine, serotonin, and endorphins.
Drugs can affect behaviour by acting as agonists by facilitating or increasing the actions of neurotransmitters, or as antagonists by blocking the action of neurotransmitters. Recreational drug use can have an effect on brain function.