
Chapter 1. Chapter 2: Chemistry and Molecules of Life
1.1 Introduction


Welcome to the Interactive Study Guide for Chapter 2: Chemistry and Molecules of Life! This Study Guide will help you master your understanding of the chapter's Driving Questions, using interactive Infographics and activities, as well as targeted assessment questions. Click "Next" to get started, or select a Driving Question from the drop-down menu to the right.
Mission to Mars:
Prospecting for life on the red planet
DRIVING QUESTIONS
- How is matter organized into molecules of living organisms?
- What is the definition of life, and how could Martian life be recognized?
- What is the basic structural unit of life?
- Why is water so important for life and living organisms?
1.2 Driving Question 1
Driving Question 1
How is matter organized into molecules of living organisms?
Why should you care?
We literally are what we eat—matter can not be created or destroyed, so every atom of every element in us was once part of some other living organism or the atmosphere or the earth. The atoms of most elements never change because the particles in them do not change. There’s a good chance that right now you may be breathing an oxygen molecule that was once breathed by Julius Caesar. You could have carbon atoms in you that were once part of a Tyrannosaurus.
What should you know?
To fully answer this Driving Question, you should be able to:
- Understand where the subatomic particles in an atom are and what their characteristics are.
- Explain why carbon is an essential chemical component of living organisms.
- Determine whether a molecule is organic or inorganic.
- Differentiate between the four classes of molecules of life and when pertinent, the subunits that comprise them.
Infographic Focus
The infographics most pertinent to the Driving Question are 2.3, 2.4, and Up Close: Molecules of Life.
Test Your Vocabulary
Choose the correct term for each of the following definitions:
| Term | Definition |
|---|---|
| (A molecule having a carbon-based backbone and at least one carbon-hydrogen bond. | |
| A positively charged subatomic particle in the nucleus of an atom. | |
| Organic molecules made up of linked nucleotide subunits; DNA and RNA are nucleic acids. | |
| Any of various (mostly) organic molecules that can bond with other molecules to form a very large molecule called a polymer. | |
| A chemically pure substance that cannot be chemically broken down; each element is made up of and defined by a single type of atom. | |
| An organic molecule made up of linked amino acid subunits. | |
| An organic molecule made up of one or more sugars. A one-sugar carbohydrate is called a monosaccharide; a carbohydrate with multiple linked sugars is called a polysaccharide. | |
| A molecule made up of individual molecules called monomers, linked together in a chain or cross-linked in two or three dimensions. | |
| The dense core of an atom. | |
| Organic molecules that generally repel water. | |
| Anything that takes up space and has mass. | |
| A group of atoms linked by covalent bonds. | |
| The building block, or monomer, of a nucleic acid. | |
| A strong chemical bond resulting from the sharing of a pair of electrons between two atoms. | |
| The smallest unit of an element that cannot be chemically broken down into smaller parts. | |
| The building block, or monomer, of a carbohydrate. | |
| electrically uncharged subatomic particle in the nucleus of an atom. | |
| (A molecule) lacking a carbon-based backbone and carbon-hydrogen bonds. | |
| The building block, or monomer, of a protein. | |
| A negatively charged subatomic particle with negligible mass; electrons orbit the nucleus. | |
| Large organic molecules that make up living organisms; they include carbohydrates, proteins and nucleic acids. |
Understand where subatomic particles in an atom are and what their characteristics are.
1.
In your notebook, draw an atom (it does not have to be of a particular element), including the subatomic particles.
- Use different shapes or colors for each kind of particle, so that you can tell them apart.
- Make sure to put the particles in the proper region of the atom.
- Add the symbols +, −, or Ø to each particle to indicate which are positively charged, negatively charged, or neutral.
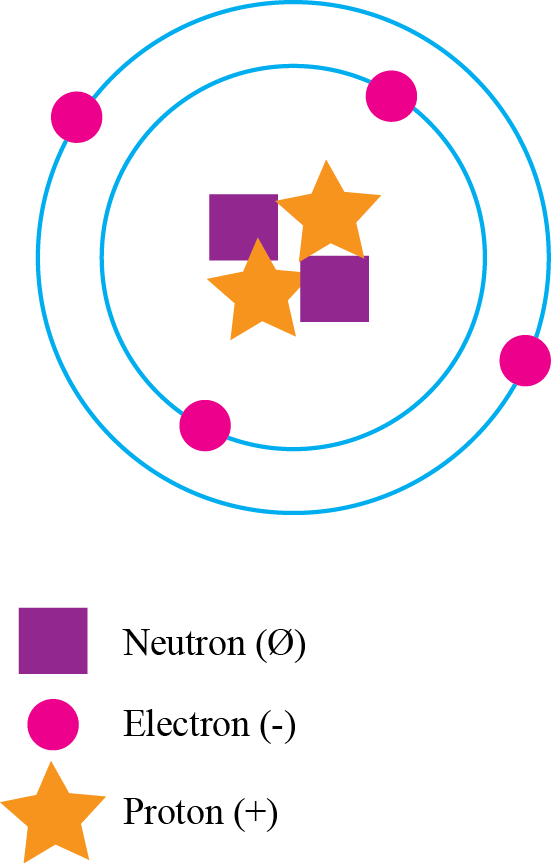
Explain why carbon is an essential chemical component of living organisms.
2.
What makes carbon such an important part of life’s molecules?
Carbon is a very versatile element in that it has four bonding sites with which to bond to other atoms through covalent bonds. Because of this flexibility, carbon forms the backbone of almost every molecule that makes up living things.
3.
What kind of chemical bonds does a carbon atom typically make with other atoms?
4.
Up to how many bonds can a carbon atom form?
Determine whether a molecule is organic or inorganic.
5.
What characterizes organic molecules?
6.
Is the compound methane (4 hydrogens attached to a central carbon) organic?
Differentiate between the four classes of molecules of life and when pertinent, the subunits that comprise them.
7.
What is the structural difference between carbohydrates, proteins, lipids, and nucleic acids, and what are they made of?
8.
For each of the following descriptions, write down which of the four molecules of life it is describing.
• when 'complex', they contain many subunits in straight or branching chains
• do not have repeating subunits
• subunits are amino acids
• some are used to speed up chemical reactions
• used for storing, transmitting, and executing genetic information
• subunits are composed of a nitrogenous base, five-carbon sugar, and phosphate group
• subunits are joined to each other with peptide bonds
• some function as hormones
• subunits are called monosaccharides
• subunits each have the same core structure but they differ in the side chains they contain
• the three-dimensional shape of the molecule is essential for it to function correctly
• some are useful as padding or insulation
• subunits usually consist of carbon rings
• the two types of molecules in this class differ by whether they consist of a single chain of subunits or two chains of subunits
• some of them are a major component of cell membranes
• subunits are nucleotides.
Review Questions
9.
The simplest kind of carbohydrate molecule is called a ____________ . Carbohydrates are important primarily because they can be used to ____________.
| A. |
| B. |
| C. |
| D. |
| E. |
10.
Which type of biological macromolecule is not made of chains of linked monomers?
| A. |
| B. |
| C. |
| D. |
| E. |
11.
All of the following are types of lipids except ___________.
| A. |
| B. |
| C. |
| D. |
| E. |
1.3 Driving Question 2
Driving Question 2
What is the definition of life, and how could Martian life be recognized?
Why should you care?
What is life? At first, the question of what is alive and what is not seems so elementary that it should not need asking. But, when it gets down to the details of what defines life, the question starts seeming a lot more profound. This chapter ponders the possibility of extraterrestrial life and how we can recognize and find it. Will it fall under the definition of life as we know it? Here on Earth, it is important to understand the nature of life, because it means understanding who we are and how we and all other life forms work.
What should you know?
To fully answer this Driving Question, you should be able to:
- List and describe 5 characteristics inherent to all living things.
- Defend an argument about whether a virus is or is not alive and how this may relate to life on Mars.
Infographic Focus
The infographics most pertinent to the Driving Question are 2.1 and 2.10.
Test Your Vocabulary
Choose the correct term for each of the following definitions:
| Term | Definition |
|---|---|
| A protein-only infectious agent. | |
| The ability to do work. Living organisms obtain energy either directly from sunlight (through photosynthesis) or from food they consume. | |
| The maintenance of a relatively constant internal environment. | |
| All of the chemical reactions taking place in the cells of a living organism that allow it to obtain and use energy. | |
| An infectious agent made up of a protein shell that encloses genetic information. |
List and describe 5 characteristics inherent to all living things.
12.

What defines life? Refer to Infographic 2.1 in your textbook if necessary.
• Growth
How does the definition of growth differ for unicellular and multicellular organisms?
13.
Why would the ability to grow be an essential quality of living organisms?
14.
• Reproduction
Why must living organisms be able to reproduce?
15.
• Homeostasis
How many ways can you think of in which you exhibit homeostasis, the quality of maintaining a stable state?
16.
Why is homeostasis an important characteristic of living things?
17.
• Sense and Respond to Stimuli:
What do you think it means to sense and respond to stimuli?
18.
What are examples of stimuli? What are the typical responses to them?
19.
• Obtain and Use Energy
What are the possible sources of energy for living organisms?
20.
Why is it essential that living things be able to use energy?
21.
The following entities are not considered alive, although they may exhibit some of the characteristics of life. For each item, explain which characteristics of life they exhibit and which ones they lack:
A rock:
22.
A computer:
Lack: Reproduction
23.
A computer virus:
Lack: growth, homeostasis, obtain and use energy
24.
The following organisms are considered alive, so they meet the five characteristics of living things. It may not be readily apparent, however, how they meet all five characteristics. For each organism below, explain why it isn't readily apparent that they are alive:
A houseplant:
25.
A wild mushroom:
26.
Mildew:
Defend an argument about whether a virus is or is not alive, and how this may relate to life on Mars.
27.
A friend asks you about viruses: Specifically she wants to know if they are alive. You explain that viruses consist of genetic material (RNA or DNA) surrounded by a protein coat, which may or may not be enclosed in a small membrane. When a virus infects a cell, the genetic material in the virus 'overwrites' the cell's genetic instructions, and the cell produces more of the components of the virus and assembles the components for it. Then, you realize you have to explain to your friend the characteristics of living organisms and whether or not a virus fits those characteristics:
Does a virus grow?
28.
Does a virus reproduce?
29.
Does a virus maintain a stable internal environment?
30.
Does a virus sense and respond to stimuli?
31.
Does a virus obtain and use energy?
32.
Keeping these characteristics in mind, what is your answer? Is a virus alive?
33.
How does your argument relate to the potential presence of life on Mars? What if you took the opposing viewpoint about viruses; how would that relate to your classification of life on Mars?
Review Questions
34.
Which of the following is not a characteristic of all living things?
| A. |
| B. |
| C. |
| D. |
| E. |
35.
True or False: Evidence of life on Mars will definitely fit within our definition of life on Earth.
| A. |
| B. |
1.4 Driving Question 3
Driving Question 3
What is the basic structural unit of life?
Why should you care?
All life on earth is made of cells, and all cells have cell membranes to hold in their contents. Cell membranes serve as boundaries, gatekeepers, and communicators. You wouldn’t be able to think, move, or generally function without proper cell membranes.
What should you know?
To fully answer this Driving Question, you should be able to:
- Describe the basic structural unit of life.
- Identify the different parts of the cell membrane.
- Explain the importance of the cell membrane.
Infographic Focus
The infographics most pertinent to the Driving Question are 2.5 and 2.10.
Test Your Vocabulary
Choose the correct term for each of the following definitions:
| Term | Definition |
|---|---|
| A phospholipid bilayer with embedded proteins that forms the boundary of all cells. | |
| The basic structural unit of living organisms. | |
| “Water-loving”, hydrophilic molecules dissolve in water. | |
| Water-fearing”, hydrophobic molecules will not dissolve in water. | |
| A type of lipid that forms the cell membrane. |
Describe the basic structural unit of life.
36.
In the most basic terms, describe the structure of a single celled organism.
37.
In the most basic terms, describe the structure of a multicellular organism.
38.
Based on your answers, why is the cell called the basic structural unit of life?
Identify the parts of the cell membrane.
39.
What are two basic components of the cell membrane?
The basic components of a cellular membrane are phospholipids and proteins.
40.
In your notebook, draw and label the typical lipid bilayer of a cell membrane.
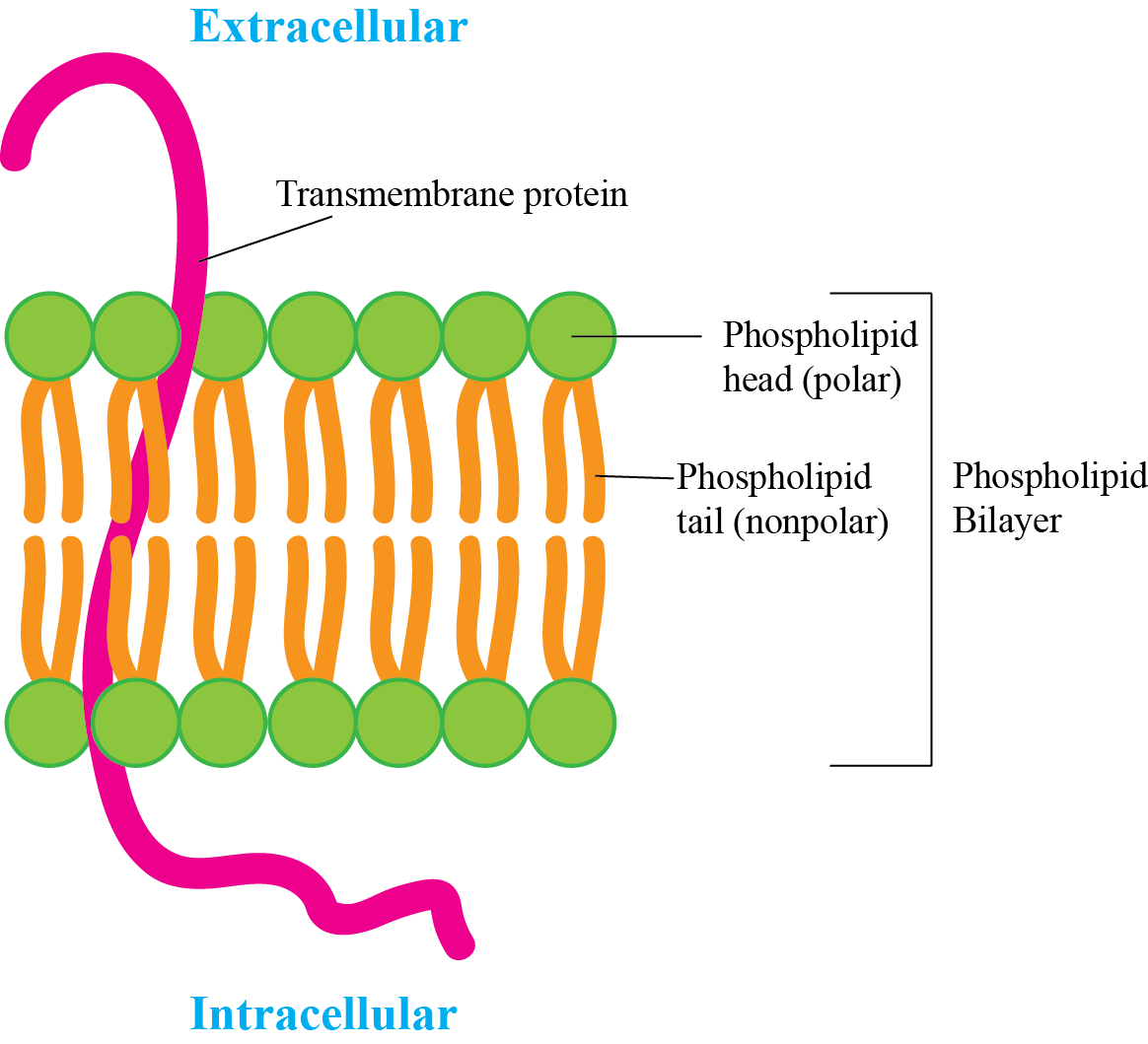
Explain the importance of the cell membrane.
41.
Why is the cell membrane important?
Review Questions
42.
The tails of phospholipids are:
| A. |
| B. |
| C. |
43.
The heads of phospholipids are:
| A. |
| B. |
| C. |
44.
What part of a phospholipid in the cell membrane interacts with the inside environment of the cell?
| A. |
| B. |
| C. |
1.5 Driving Question 4
Driving Question 4
Why is water so important for life and living organisms?
Why should you care?
You might rightly say that a sixth characteristic of living things is the presence of water in them. Your body contains around 60% water by weight. All living and actively growing things are made of cells containing mostly water. How did water get to be so important to life? It has several unique characteristics that make it a special molecule.
Because it is a polar molecule (with unevenly distributed charges across it), water is a good solvent for charged or polar substances. Water can break ionic bonds in solutes like salt, so that its component ions are released into the solution as charged particles. The movement of ions in water solution is essential for your brain and muscles to work. Nearly all of the complex chemical interactions that go on every moment of your life—and that enable you to think, live, and breathe—happen in water. The polarity of water molecules is also important in the formation of hydrogen bonds with each other and with other polar biomolecules; this in turn helps keep your DNA together in your cells and your proteins in their proper shapes. Furthermore, you can’t taste or smell organic molecules like sugar or the scent of lemons until they dissolve in water.
What should you know?
To fully answer this Driving Question, you should be able to:
- Diagram a water molecule and label its components.
- Explain why water is a polar molecule and why this makes it a good solvent for charged or polar substances.
- Explain what hydrogen bonding is and its implications for the properties of water.
- Explain why water is important for life.
Infographic Focus
The infographics most pertinent to the Driving Question are 2.5, 2.6, 2.7, 2.8 and 2.9.
Test Your Vocabulary
Choose the correct term for each of the following definitions:
| Term | Definition |
|---|---|
| A substance that increases the hydrogen ion concentration of solutions, making them more acidic. | |
| A weak electrical attraction between a partially positive hydrogen atom and an atom with a partial negative charge. | |
| A strong electrical attraction between oppositely charged ions formed by the transfer of one or more electrons from one atom to another. | |
| A substance that reduces the hydrogen ion concentration of solutions, making them more alkaline. | |
| (Water) molecules sticking to (water) molecules through hydrogen bonding. | |
| A molecule in which electrons are not shared equally between atoms, causing a partial negative charge at one end and a partial positive charge at the other; for example, water. | |
| A dissolved substance. | |
| A measure of the concentration of hydrogen ions in a solution. | |
| (Water) molecules sticking to other surfaces through hydrogen bonding. | |
| An electrically charged atom, the charge resulting from the loss or gain of electrons. | |
| The mixture of solute and solvent. | |
| A substance in which other substances can dissolve; for example, water. |
Diagram a water molecule and label its components.
45.
In your notebook, draw and label a diagram of a water molecule. Make sure to label where the positive and negative charges are on the molecule.
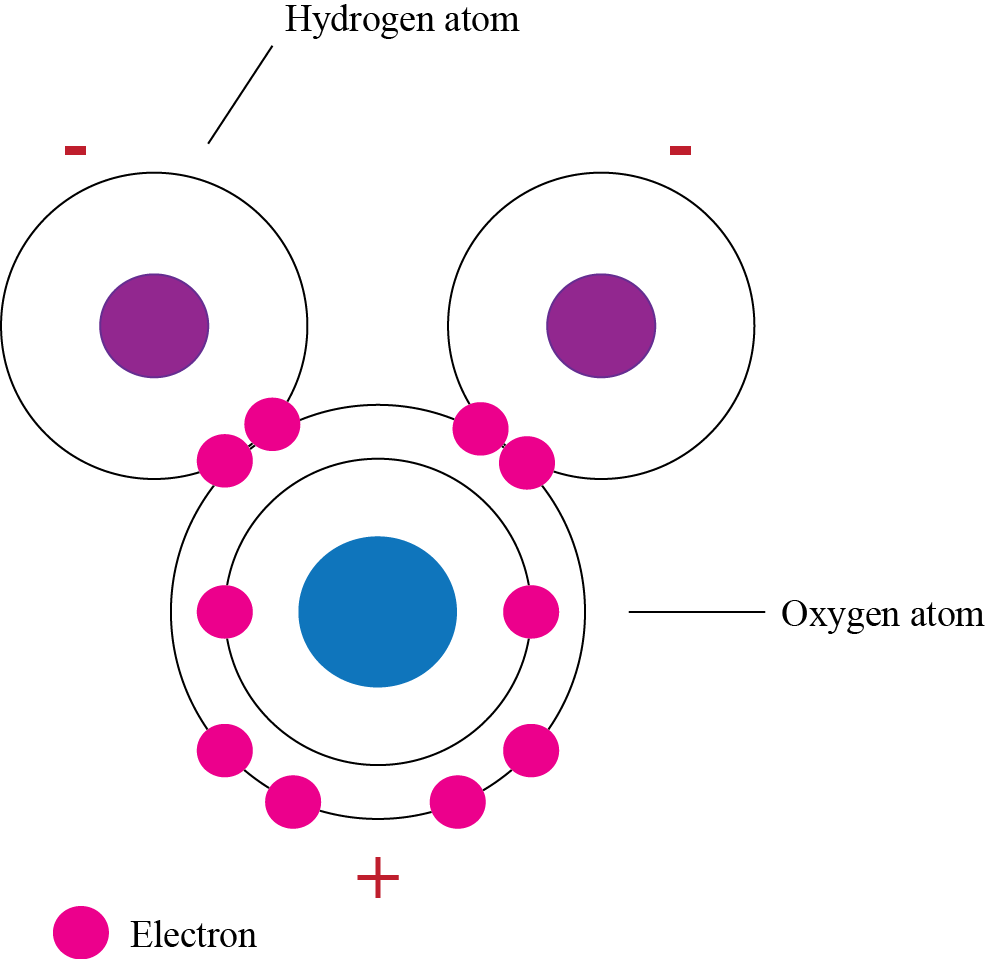
Explain why water is a polar molecule and why this makes it a good solvent for charged or polar substances.
46.
Are the atoms in water covalently or ionically bonded? How do you know?
47.
Why is water a polar molecule?
48.
What does being polar have to do with being a good solvent?
49.
If you dissolve the salt magnesium bromide (MgBr2) in water, the magnesium atoms and the bromine atoms dissociate from each other as positively-charged magnesium ions (Mg2+) and negatively charged bromide ions (Br1-). When water molecules surround the magnesium ions, what side of each water molecule will be directed towards them, the hydrogen or oxygen side?
Explain what hydrogen bonding is and what implications it has for the properties of water.
50.
What is hydrogen bonding and how does it affect water bonding with itself and other substances?
51.
How does hydrogen bonding affect the heat capacity of water?
Explain why water is important for life.
52.
Based on your answers to the previous questions, why is water important for life?
Review Questions
53.
Water molecules attracted to each other by hydrogen bonds will be connected by bonds between ____________.
| A. |
| B. |
| C. |
| D. |
| E. |
54.
Which of the following pairs of terms apply to a single water molecule’s structure?
| A. |
| B. |
| C. |
| D. |
55.
Waxes are a kind of lipid. Why does water form beads on wax paper?
| A. |
| B. |
| C. |
| D. |
Activity results are being submitted...