5-2 Earth is surrounded by a thin, multilayered envelope of gas that has changed since life became prominent
As we look around the solar system at our nearest planetary neighbors, Venus and Mars, we notice that these planets have a relatively thin layer of protective gas surrounding them. This gaseous envelope of air surrounding a planet or moon is called an atmosphere. While Venus has an incredibly dense atmosphere, Mars has an atmosphere of very low density. Moreover, an analysis of sunlight reflected by the atmospheres of Venus and Mars shows that their air is largely carbon dioxide (CO2). So, it might be somewhat surprising to learn that we see something quite different when we consider what makes up Earth’s air. Our air is about 78% nitrogen and 21% oxygen, with the remaining mostly composed of water vapor, argon gas, and carbon dioxide (Figure 5-3). This is quite different than Venus and Mars.

Earth’s original CO2 atmosphere was much like other planets until the emergence of life dramatically changed it.
We suspect that most planets’ atmospheres initially formed in the same way. When planets like Earth first formed by accretion of planetesimals, as described in Section 4-6, gases were trapped within Earth’s interior in the same proportions that they were present in the solar nebula. But since the early Earth was hot enough to be molten throughout its volume, most of these trapped gases eventually leaked into space, easily able to escape Earth’s gravity. The atmosphere that remained still contained substantial amounts of hydrogen, but in the form of relatively massive molecules of water vapor (H2O) made by combining two atoms of hydrogen with one atom of oxygen, the third most common element in our part of the Milky Way Galaxy. In fact, water vapor and carbon dioxide were probably the dominant constituents of the early atmosphere, just like on Venus and Mars today. Somehow our atmosphere changed substantially since the planets were formed about 4.56 billion years ago.
Emerging Life’s Impact on Earth’s Atmosphere
Carbon dioxide would have been released into Earth’s early atmosphere by erupting volcanoes and much of it would have been trapped within rocks. Carbon dioxide dissolves in rainwater and falls into the oceans, where it can combine with other substances to form a category of rocks known as carbonates, which form layers on the ocean floor. (Limestone and marble are examples of carbonate-bearing rock.) The end result is that the amounts of CO2 that we see in the air today are the result of a natural balance between erupting volcanoes that release CO2 into the atmosphere and the formation of carbonates that naturally remove CO2 from the air.
The appearance of life on Earth set into motion a radical transformation of the atmosphere. Early single-cell organisms converted energy from sunlight into chemical energy using photosynthesis, a chemical process that consumes CO2 and water and releases oxygen (O2). As life proliferated and evolved on Earth, the amount of photosynthesis increased dramatically. Eventually O2 began to accumulate in the atmosphere. Figure 5-4 illustrates how the amount of O2 in the atmosphere has increased over the history of Earth.
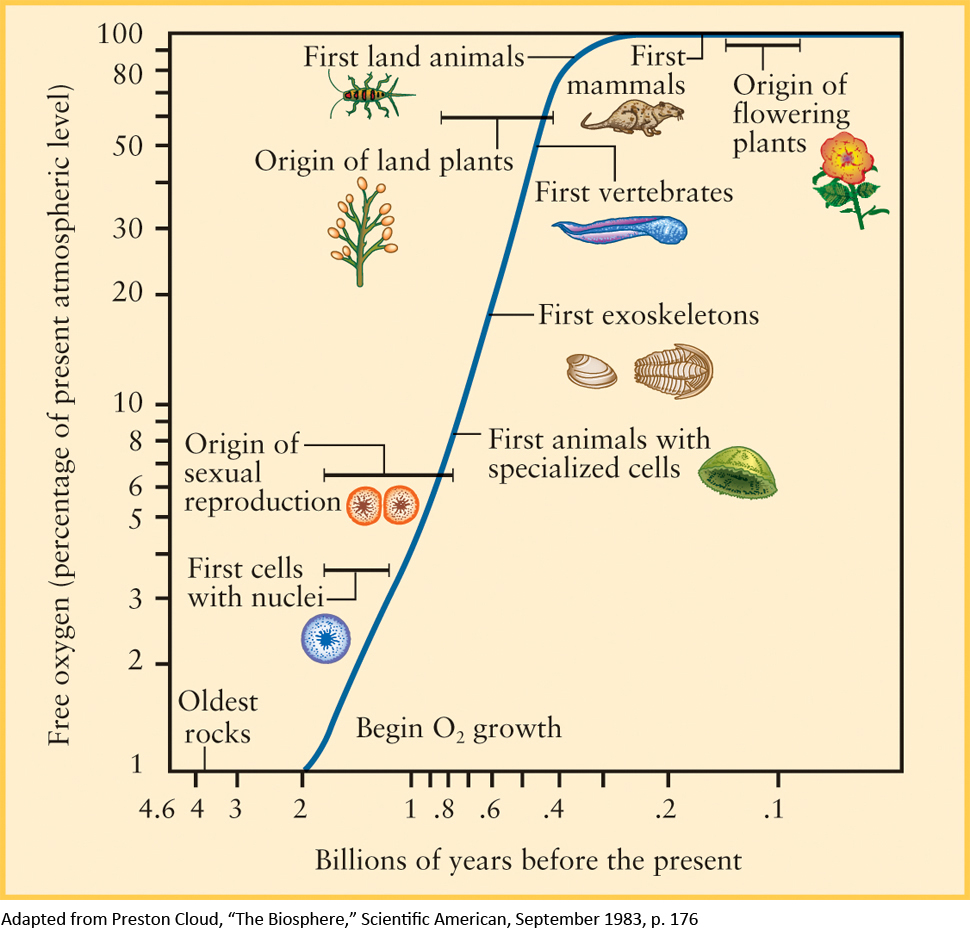
About 2 billion (2 × 109) years ago, a new type of life evolved to take advantage of the newly abundant oxygen. These new organisms produce energy in a process very different from photosynthesis. Instead, these newly abundant life-forms consume oxygen and release carbon dioxide using a process called respiration. Respiration is the same process used by all modern animals, including humans. Oxygen-breathing organisms thrived because photosynthetic plants continued to add even more oxygen to the atmosphere.
119
Several hundred million years ago, the number of oxygen molecules in the atmosphere stabilized at 21% of the total, the same as the present-day value. This value represents a balance between the release of oxygen from plants by photosynthesis and the absorption of oxygen by animals, bacteria, and rocks. The role of life in the production of oxygen in the atmosphere is so important that when they look at planets beyond our own, astronomers look for evidence of oxygen as a hint that life might be present on that planet.
The most numerous molecules in our atmosphere are nitrogen (N2), which make up 78% of the total. These, too, are a consequence of the presence of life on Earth. Certain bacteria use an energy generation process that extracts oxygen from chemicals called nitrates, and in the process nitrogen is released into the atmosphere. Volcanoes outgassing ammonia and ammonia’s subsequent dissociation are another source of atmospheric nitrogen. Interestingly, the amount of atmospheric nitrogen is kept in check not by another living entity but by lightning occurring during thunderstorms. It turns out that the sudden burst of energy in a lightning flash causes nitrogen and oxygen in the air to combine into nitrogen oxides, which dissolve in rainwater, fall into the oceans, and form the nitrates that bacteria use to produce energy. What this means is that Earth not only relies on a water cycle, which we saw in the previous section, but there is also a continuous carbon cycle and a continuous nitrogen cycle running on Earth.
Question
ConceptCheck 5-3: What life process converts a predominately carbon dioxide atmosphere to a predominately nitrogen and oxygen atmosphere?
Pressure, Temperature, and Convection in the Atmosphere
We have looked at the contents of Earth’s atmosphere, but one wonders how the atmosphere changes as you get farther away from Earth’s surface. Imagine smoothly drifting upward in a hot air balloon. As you go up in the atmosphere, there is less and less air pressing down on you from above. Hence, the amount of air above you decreases with increasing altitude. Atmospheric pressure at any height in the atmosphere is caused by the weight of all the air above that height. The average atmospheric pressure at sea level is defined to be 1 atmosphere (1 atm), equal to 1.01 × 105 N/m2 or 14.7 pounds per square inch.
At the same time, in our imaginary balloon ride, you would notice that the temperature also changes. However, you might be surprised to observe that, unlike atmospheric pressure, temperature varies with altitude in a complex way. Figure 5-5 shows that temperature decreases with increasing altitude in some layers of the atmosphere, but in other layers actually increases with increasing altitude. These differences result from the individual ways in which each layer is heated and cooled.
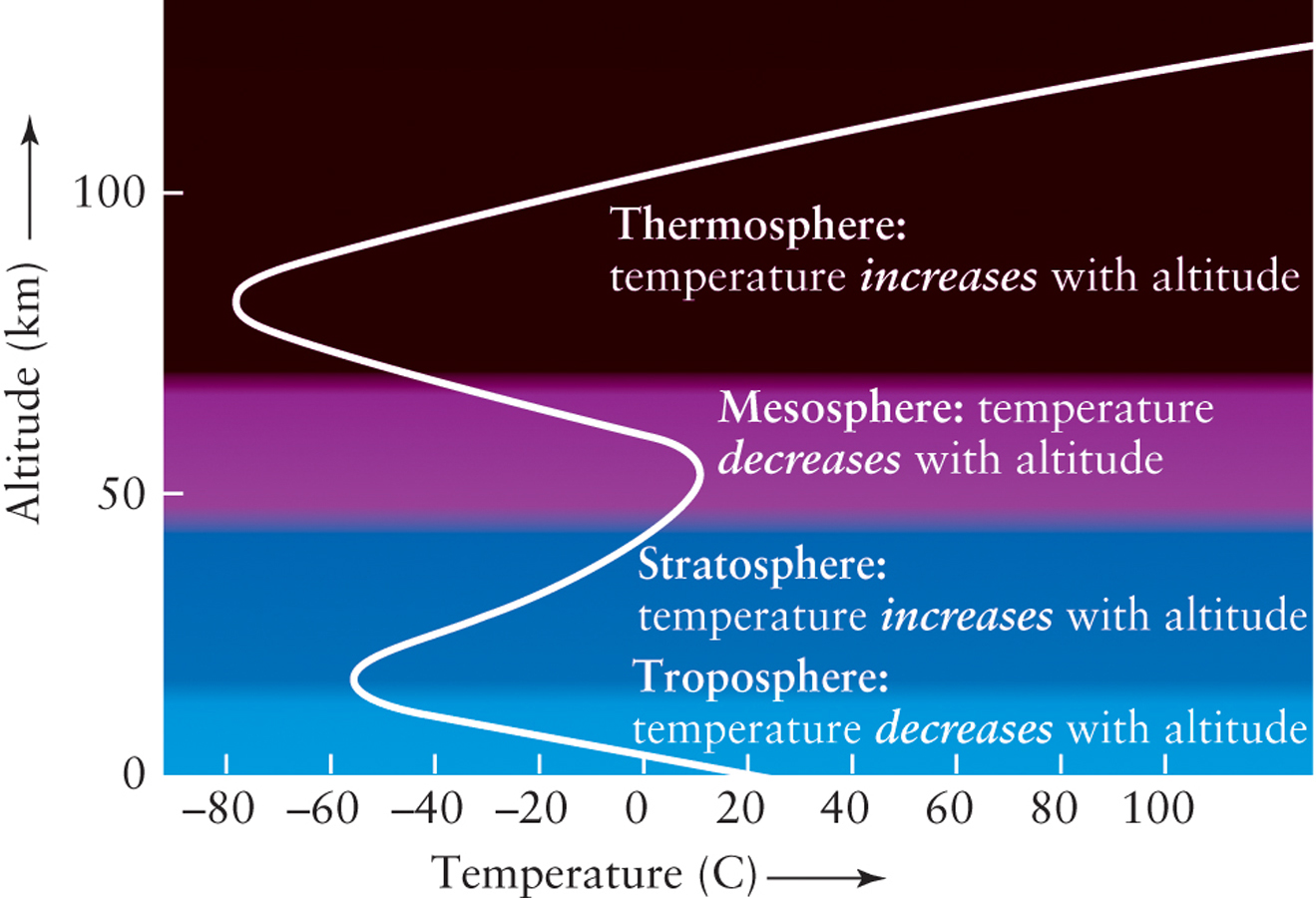
The lowest layer, called the troposphere, extends from the surface to an average altitude of 7.5 mi (roughly 39,000 ft or 12 km). This region closest to Earth’s surface is where most of Earth’s weather and clouds are found. This part of the atmosphere is heated by energy emitted by Earth’s Sun-warmed surface, rather than directly by the Sun itself. Sunlight efficiently warms Earth’s surface, which heats the lowest part of our atmosphere. By contrast, the upper part of the troposphere has cooler temperatures because the air there is farther from Earth’s warming surface. The movement of air and water vapor in this region is what causes much of Earth’s weather.
120
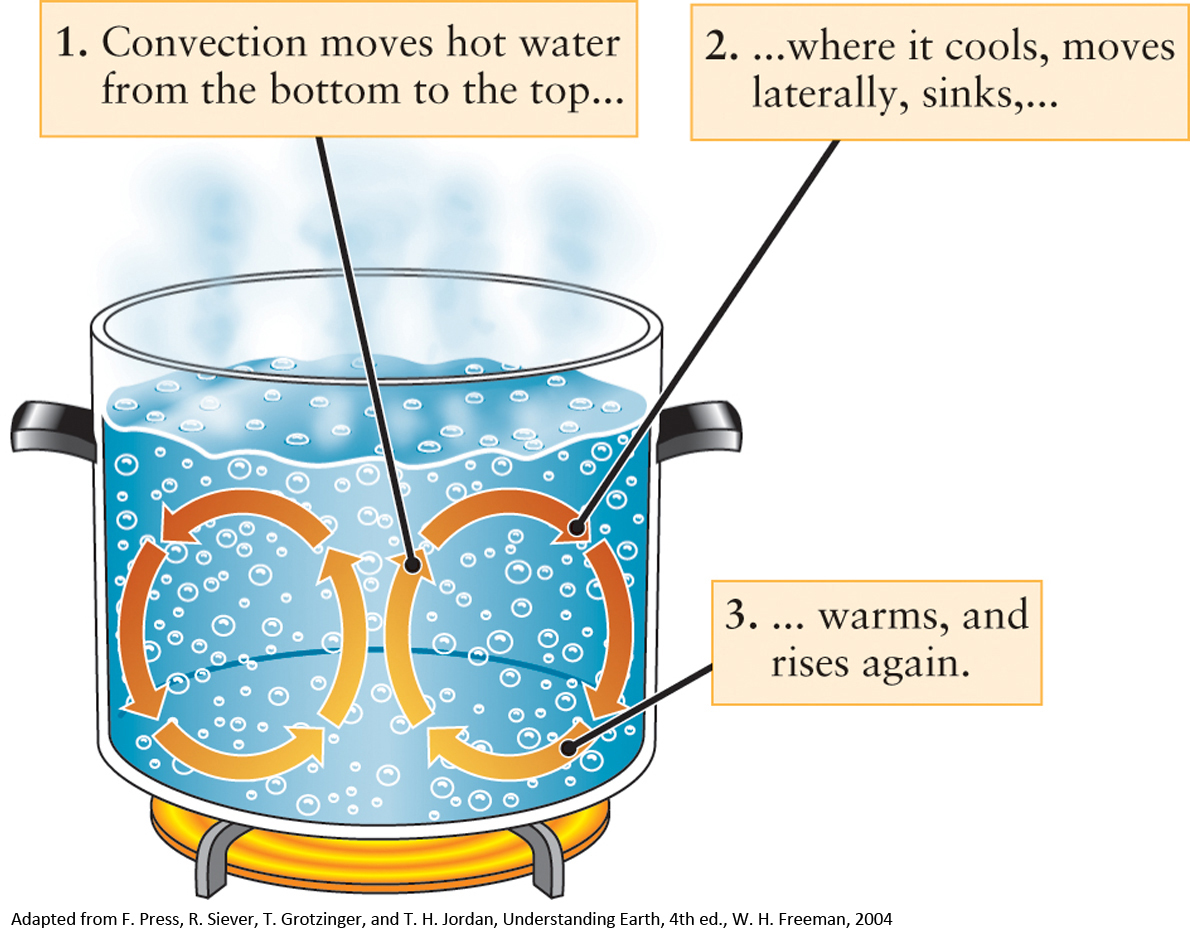
Low-altitude air warmed by Earth’s surface expands and becomes less dense. Because this air is now less dense than the cooler air above it, the warm air starts to rise, pushing the cooler air down toward the surface in the process. Of course, that cooler air is now able to be heated by Earth’s Sun-warmed surface and it becomes less dense and moves upward. The previously warmed air now cools and contracts because it has moved away from Earth’s warming surface and begins to descend, and the process starts all over again. This cycling process of warm air rising and cool air falling over and over again is known as convection. It is the same process that helps water to churn over and over in a boiling pot of water, as illustrated in Figure 5-6. Convection is a common process throughout the universe that we will encounter again and again: Convection accounts for the movement of energy within Earth and for how some energy generated in the cores of stars makes its way to the surface.
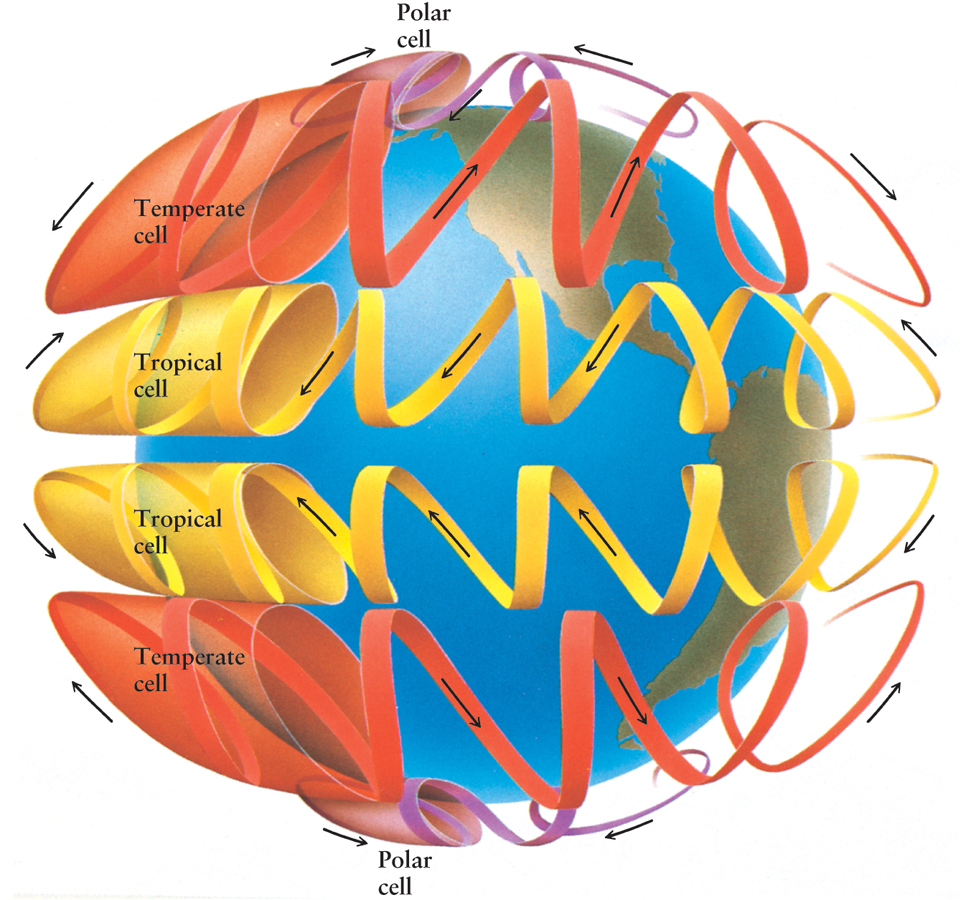
Convection on a grand scale is caused by the temperature difference between Earth’s equator and its poles. If Earth did not rotate, heated air near the equator would rise upward and flow at high altitude toward the poles. There it would cool and sink to lower altitudes, at which it would flow back to the equator. However, Earth’s rotation breaks up this simple convection pattern into a series of smaller convection circles. In these smaller circles, air flows east and west as well as vertically and in a north-south direction. The structure of these cells explains why the prevailing winds blow in different directions at different latitudes (Figure 5-7).
Question
ConceptCheck 5-4: Is the atmospheric layer called the troposphere heated from above or below?
Upper Layers of the Atmosphere
Almost all the oxygen in the troposphere is in the form of O2, a molecule made of two oxygen atoms. But in the stratosphere, which extends from about 7.5 mi to 31 mi (about 12 km to 50 km) above the surface, some oxygen is in the form of ozone, a molecule made of three oxygen atoms (O3). Ozone is very efficient at absorbing potentially harmful ultraviolet radiation from the Sun, which means that the stratosphere can directly absorb solar energy. The result is that the temperature actually increases, rather than decreases, as you move upward in the stratosphere. Convection requires that the temperature must decrease, not increase, with increasing altitude, so there are essentially no convection currents in the stratosphere.
Above the stratosphere lies the mesosphere. Very little ozone is found here, so solar ultraviolet radiation is not absorbed within the mesosphere, and atmospheric temperature again declines with increasing altitude as cooling occurs. The temperature of the mesosphere reaches a minimum of about −5°C (= −103°F = 198 K) at an altitude of about 50 mi (80 km). This minimum marks the bottom of the atmosphere’s thinnest and uppermost layer, the thermosphere, in which temperature once again rises with increasing altitude. This is not due to the presence of ozone, because in this very low–density region oxygen and nitrogen are found mostly as individual atoms rather than in molecules. Instead, the thermosphere is heated because these isolated atoms absorb very-short-wavelength solar ultraviolet radiation, which oxygen and nitrogen molecules are not plentiful enough to absorb.
121
CAUTION
At altitudes near 200 mi above Earth’s surface the temperature of the thermosphere is about 1000°C (1800°F). This is near the altitude at which the space shuttle and satellites orbit Earth. Nonetheless, a satellite in orbit does not risk being burned up as it moves through the thermosphere. The reason is that the thermosphere is far less dense than the atmosphere at sea level. The high temperature simply means that an average atom in the thermosphere is moving very fast. But because the thermosphere is so thin (only about 10−11 as dense as the air at sea level), these fast-moving atoms are few and far between. Hence, the thermosphere contains very little energy. Nearly all the heat that an orbiting satellite receives is from sunlight, not the thermosphere.
Question
ConceptCheck 5-5: What would happen to the temperature in the stratosphere if there was an absence of ozone?
The Sun’s Role in the Atmosphere’s Energy
The Sun is the principal source of energy for the atmosphere. Earth’s surface is warmed by sunlight, which in turn warms the air next to the surface. Solar energy also powers weather systems when water evaporates from the surface. The energy in the water vapor is released when it changes back into water from water droplets, like those that make up clouds. In a typical thunderstorm, some 5 × 108 kg of water vapor is lifted to great heights. The amount of energy released when this water condenses is as much energy as a city of 100,000 people uses in a month!
Solar energy also helps to power the oceans. Warm water from near the equator moves toward the poles, while cold polar water returns toward the equator. In the end, the average surface temperature of Earth depends almost entirely on the amount of energy that reaches us from the Sun in the form of electromagnetic radiation. In an analogous way, whether you feel warm or cool outdoors on a summer day depends on whether you are in the sunlight and receiving lots of solar energy or in the shade and receiving little of this energy.
One might think that if Earth did nothing but absorb radiation from the Sun, it would get hotter and hotter until the surface temperature became high enough to melt rock. Happily, there are at least two important reasons why this could not happen. One is that the clouds, snow, ice, and sand reflect about 31% of the incoming sunlight back into space. The fraction of incoming sunlight that a planet reflects is called its albedo (from the Latin for “whiteness”); thus, Earth’s albedo is about 0.31. In other words, only 69% of the incoming solar energy is absorbed by Earth and the rest bounces back into space. A second reason is that Earth also emits radiation into space itself because of its own temperature, in accordance with the laws that describe energy emitted by dense objects (see Section 2-3). Earth’s average surface temperature is nearly constant, which means that on the whole it is neither gaining nor losing energy.
To better understand this balance between absorbed and emitted radiation, remember that Wien’s law tells us that the wavelength at which such an object emits most strongly (λmax) is inversely proportional to its temperature (T) on the Kelvin scale (see Chapter 2). For example, the Sun’s surface temperature is about 5800 K, and sunlight has its greatest intensity at a wavelength λmax of 500 nm, in the middle of the visible spectrum. Earth’s average surface temperature of 287 K is far lower than the Sun’s, so Earth radiates most strongly at longer wavelengths in the infrared portion of the electromagnetic spectrum. The Stefan-Boltzmann law (see Chapter 2) tells us that temperature also determines the amount of radiation that Earth emits: The higher the temperature, the more energy it radiates.
Given the amount of energy reaching us from the Sun each second as well as Earth’s albedo, we can calculate the amount of solar energy that Earth should absorb each second. Since this must equal the amount of electromagnetic energy that Earth emits each second, which in turn depends on Earth’s average surface temperature, we can calculate what Earth’s average surface temperature should be. The result is a very chilly 254 K (= −19°C = −2°F), so cold that oceans and lakes around the world should be frozen over. In fact, Earth’s actual average surface temperature is 287 K (= 14°C = 57°F). What is wrong with our model? Why is Earth warmer than we would expect?
The explanation for this discrepancy is called the greenhouse effect: Our atmosphere prevents some of the radiation emitted by Earth’s surface from escaping into space. This process happens on any planet or moon that has an atmosphere. On Earth, certain gases in our atmosphere, called greenhouse gases, among them water vapor and carbon dioxide, are transparent to visible light but not to infrared radiation. Consequently, visible sunlight has no trouble entering our atmosphere and warming the surface. But the infrared radiation coming from the heated surface is partially absorbed by the atmosphere, thus raising the temperatures of both the atmosphere and the surface. As the surface and atmosphere become hotter, they both emit more infrared radiation, part of which is able to escape into space. The temperature levels off when the amount of infrared energy that escapes just balances the amount of solar energy reaching the surface (Figure 5-8). The result is that our planet’s surface is some 33°C (59°F) warmer than it would be without the greenhouse effect, and water remains unfrozen over most of Earth.
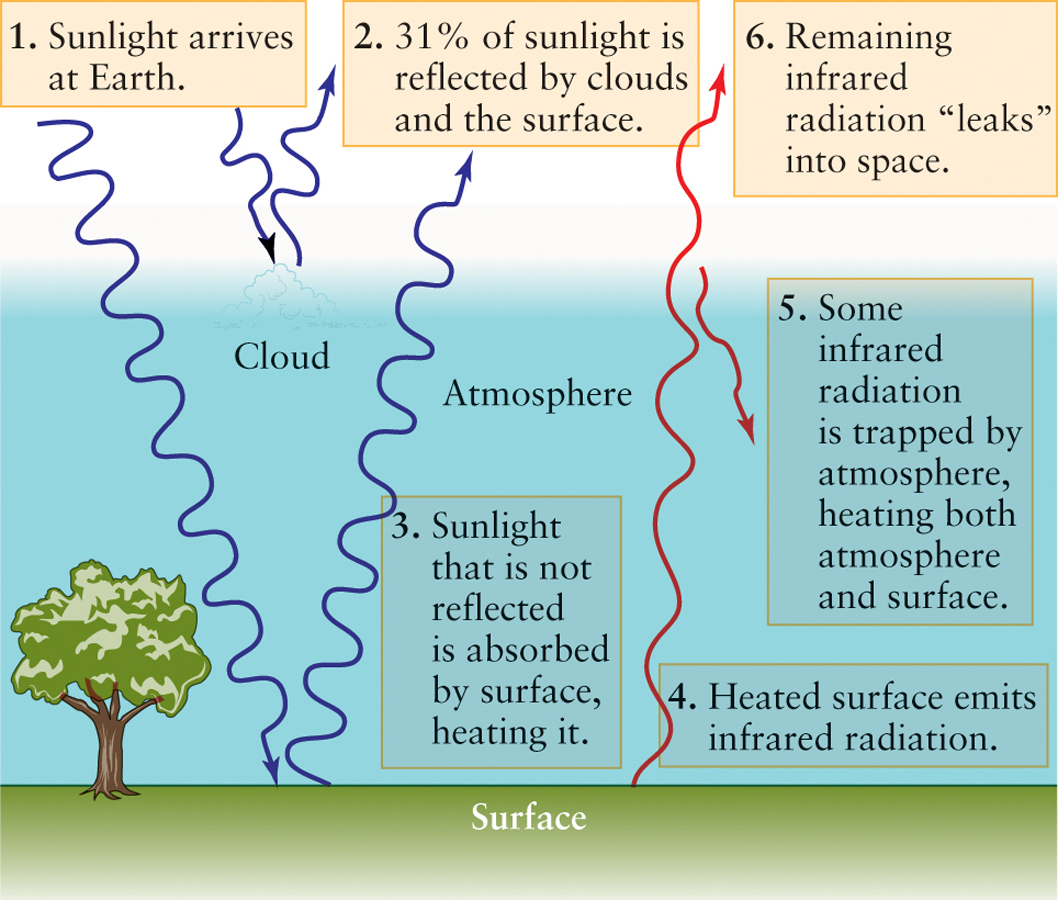
The warming caused by the greenhouse effect helps to give our planet the moderate temperatures needed for the existence of life. For more than a century, however, our technological civilization has been adding greenhouse gases to the atmosphere at an unprecedented rate. In this instance, it is difficult to predict what the effects of this imbalance might be.
Question
ConceptCheck 5-6: What is the positive impact that the greenhouse effect has on our planet?
122