6-4 Atmospheres surrounding terrestrial planets vary considerably
We have talked about Earth having a special place in the solar system—not too close to the Sun, not too far, but just right. So, what is “just right” about Earth’s position as a planet where life can flourish? In short, Earth’s position and protective atmosphere of gas surrounding the planet stabilizes the liquid water that life requires to exist. If Earth were any closer to the Sun, then any water would likely boil away; and if Earth were farther away, then any water would likely freeze. If planets nearer and farther away from the Sun have atmospheres so different from Earth, what are they like? Mercury has a daytime temperature too high and gravity too weak to retain any substantial atmosphere, so it will not get our attention here. We have seen that the three large terrestrial planets—Earth, Venus, and Mars—display very different types of geologic activity; as we will discover, these differences help to explain why each of these three worlds has a distinctively different atmosphere. However, there are also similarities.
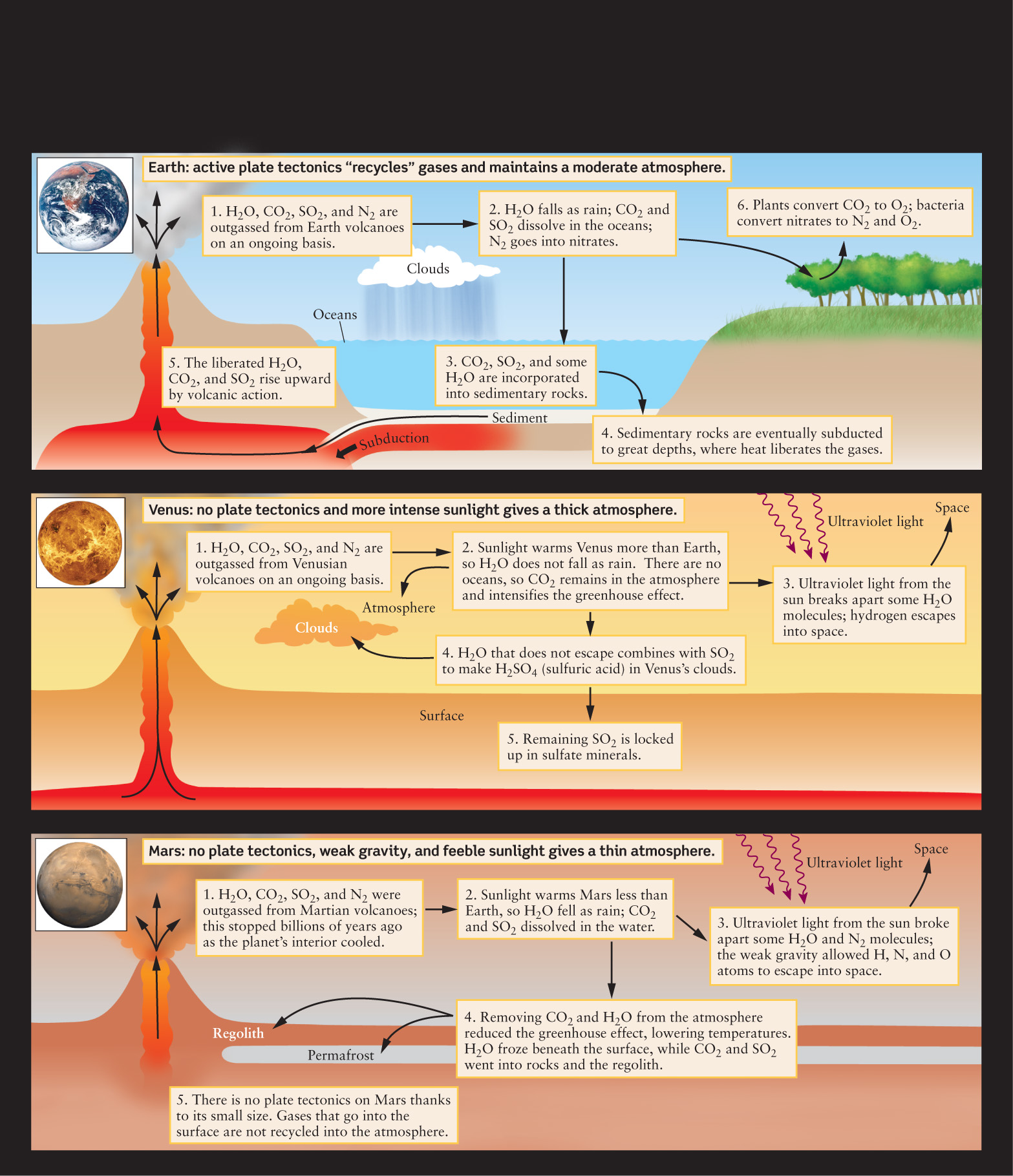
The original atmospheres of Earth, Venus, and Mars all derived from gases that were emitted, or outgassed, from volcanoes (Figure 6-17). The gases released by present-day Earth volcanoes are predominantly water vapor (H2O), carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen (N2). These gases should therefore have been important parts of the original atmospheres of all three planets. Much of the water on all three planets is thought to have come from impacts from comets, icy bodies from the outer solar system.

In order to better understand the various atmospheres found around the solar system, let’s first consider some of the most important aspects of Earth’s atmosphere.
- Earth’s atmosphere is predominately composed of nitrogen. There are certainly some water molecules in Earth’s atmosphere—most easily seen as clouds—but most of Earth’s water is found in the oceans.
- Similarly, some carbon dioxide is found in Earth’s atmosphere, but much of Earth’s carbon dioxide has been captured in carbonate rocks, such as limestone. This occurs because carbon dioxide readily dissolves in water, so rain can remove CO2 from the atmosphere, which is ultimately deposited on ocean floors where it cements into rocks.
- The oxygen (O2) in Earth’s atmosphere is the result of photosynthesis by plant life, which absorbs CO2 and releases O2. The net result is that our planet’s atmosphere is predominantly N2 and O2, with enough pressure and a high enough temperature (thanks to the greenhouse effect) for water to remain a liquid—and hence for life, which requires liquid water, to exist.
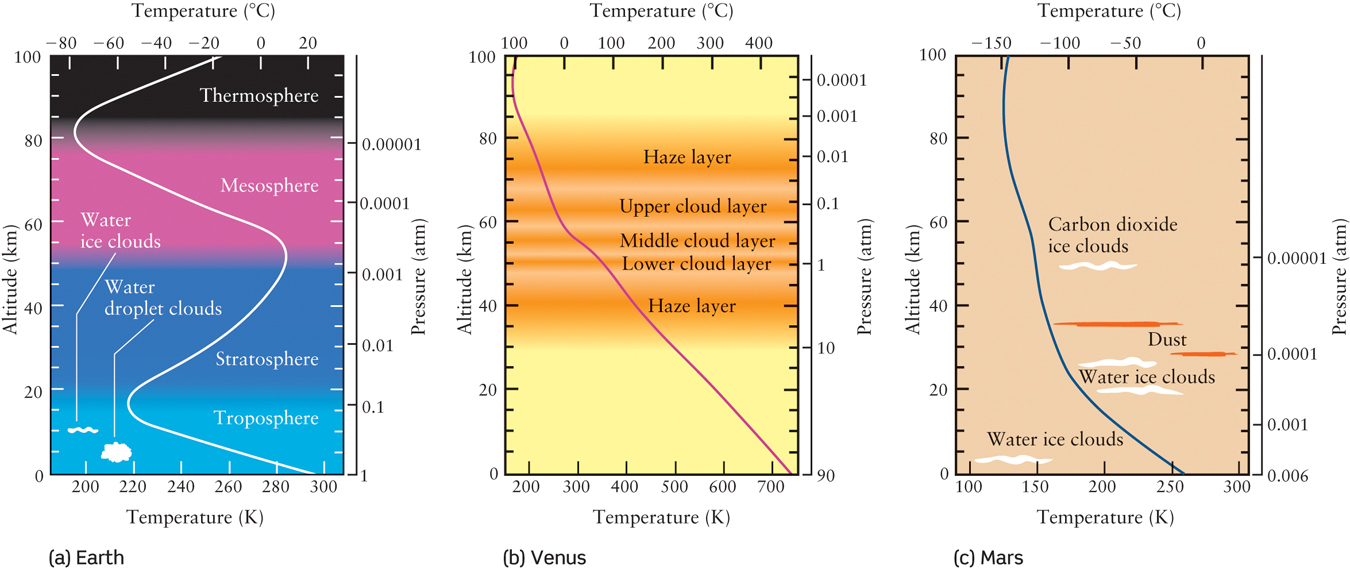
In order to compare the atmospheres of Venus and Mars to Earth’s, it is necessary to get information from spacecraft that are able to descend through an atmosphere to take measurements. Both Soviet and U.S. spacecraft have done this for Venus, and five U.S. spacecraft have successfully landed on Mars. The results of these missions are summarized in Figure 6-18, which shows how pressure and temperature vary with altitude in the atmospheres of Earth, Venus, and Mars.
156
Close-up Observations of Venus Reveal a Broiled Planet
Venus is bathed in more intense sunlight than Earth because it orbits closer to the Sun than Earth does. If Venus had no atmosphere, and if its surface reflected sunlight back into space like that of Mercury or the Moon, heating by the Sun would bring its surface temperature to around 113°F (45°C)—comparable to that found in the hottest regions on Earth. However, what is surprising is that Venus is many times hotter than this. Venus’s temperatures are well above the boiling point of water and higher even than daytime temperatures on Mercury, which is closest to the Sun and most of whose atmosphere was boiled away billions of years ago.
Most terrestrial planetary atmospheres are dominated by CO2, unless altered by biological activity or removed by the solar wind.
When Venus was young and had liquid water, the amount of atmospheric CO2 was kept at small and stable levels, much as occurs on Earth today: CO2 released by volcanoes quickly dissolved in the oceans and was then bound up in carbonate rocks. Enough of the liquid water would have vaporized to create a thick cover of water vapor clouds. Since water vapor is a greenhouse gas, this humid atmosphere—perhaps denser than Earth’s present-day atmosphere, but far less dense than the atmosphere that surrounds Venus today—would have efficiently trapped heat from the Sun. At first, this would have had little effect on the oceans of Venus. Although the temperature would have climbed above 212°F (100°C), the boiling point of water at sea level on Earth, the added atmospheric pressure from water vapor would have kept the water in Venus’s oceans in the liquid state.
This hot and humid state of affairs may have persisted for several hundred million years. But as the Sun’s energy output slowly increased over time, the temperature at the surface would eventually have risen above 700°F (647 K or 374°C). Above this temperature, no matter what the atmospheric pressure, Venus’s oceans would have begun to evaporate. The added water vapor in the atmosphere would have increased the greenhouse effect, making the temperature even higher and causing the oceans to evaporate faster. That would have added even more water vapor to the atmosphere, further intensifying the greenhouse effect and making the temperature climb higher still. This is an example of a runaway greenhouse effect, in which an increase in temperature causes a further increase in temperature, and so on.
ANALOGY
A runaway greenhouse effect is like a house in which the thermostat has accidentally been connected backward. If the temperature in such a house gets above the value set on the thermostat, the heater comes on and makes the house even hotter.
Today, Venus is dramatically different from what it was in the past, and it isn’t like any of the other planets. Venus has a dry, desertlike surface with no oceans, lakes, or rivers. The infrared-absorbing properties of CO2 have stabilized Venus’s surface temperature at its present value of 860°F (460°C). Only minuscule amounts of water vapor—about 30 parts per million, or 0.003%—remain in the atmosphere. As on Earth, volcanic outgassing still adds small amounts of water vapor to the atmosphere, along with carbon dioxide and sulfur dioxide. But on Venus, these water molecules either combine with sulfur dioxide to form sulfuric acid clouds or break apart due to solar ultraviolet radiation. The great irony is that this state of affairs is the direct result of an earlier Venusian atmosphere that was predominantly water vapor.
157
Figure 6-18b depicts just how dense the Venusian atmosphere is. At the surface, the pressure is 90 atm—that is, 90 times greater than the average air pressure at sea level on Earth. This is about the same as the water pressure at a depth of about half a mile below the surface of Earth’s oceans. Venus’s atmosphere is considerably denser, too. The density of the atmosphere at the surface of Venus is more than 50 times greater than the density of our atmosphere at sea level. The atmosphere is incredibly massive and does not change easily. In fact, once heated by the Sun, the atmosphere retains its heat throughout the long Venusian night. As a result, temperatures on the day and night sides of Venus are almost identical.
Soviet and U.S. spacecraft also discovered that Venus’s clouds are primarily confined to three high-altitude layers. An upper cloud layer lies at altitudes between 42 mi and 36 mi (68 km and 58 km), a denser and more opaque cloud layer from 36 mi to 32 mi (58 km to 52 km), and an even denser and more opaque layer between 32 mi and 30 mi (52 km and 48 km). Above and below the clouds are 20-km-thick layers of haze. Below the lowest haze layer, the atmosphere is remarkably clear all the way down to the surface of Venus.
Sulfur plays an important role in the Venusian atmosphere. Sulfur combines with other elements to form gases such as sulfur dioxide (SO2) and hydrogen sulfide (H2S), along with sulfuric acid (H2SO4), the same acid used in automobile batteries. While Earth’s clouds are composed of water droplets, Venusian clouds contain almost no water. Instead, they are composed of droplets of concentrated, corrosive sulfuric acid. Thanks to the high temperatures on Venus, these droplets never rain down on the planet’s surface; they simply evaporate at high altitude. (You can see a similar effect on Earth. On a hot day in the desert of the U.S. Southwest, streamers of rain called virga appear out of the bottoms of clouds but evaporate before reaching the ground.)
On Earth, friction between the atmosphere and the ground causes wind speeds at the surface to be much less than at high altitude. The same is true on Venus: The greatest wind speed measured by spacecraft on Venus’s surface is only about 3 mi/h (5 km/h). Thus, only slight breezes disturb the crushing pressures and infernal temperatures found on Venus’s dry, lifeless surface. Whereas Venus has an incredibly thick atmosphere compared to Earth’s, observations of Mars show that Mars has an atmosphere far thinner.
Cosmic Connections: Evolution of Terrestrial Atmospheres summarizes the processes that led to the present-day atmospheres of the three large terrestrial planets.
Question
ConceptCheck 6-10: What is “runaway” about Venus’s runaway greenhouse?
The Martian Atmosphere: Not a Drop to Drink
 Go to Video 6-4
Go to Video 6-4
Observations show that Mars has far less atmosphere than either Venus or Earth, but it might not always have been that way. Mars probably had a thicker atmosphere 4 billion years ago. Thanks to Mars’s greater distance from the Sun and hence less intense sunlight, temperatures would have been lower than on the young Earth and any water in the atmosphere would more easily have fallen as rain or snow. This would have washed much of the planet’s carbon dioxide from its atmosphere, perhaps creating carbonate minerals in which the CO2 is today chemically bound. Measurements from Mars orbit show only small amounts of carbonate materials on the surface, suggesting that that the amount of atmospheric CO2 that rained out was small. Hence, even the original Martian atmosphere was relatively thin, though thicker than the present-day atmosphere.
Because Mars is so small, it cooled early in its history and volcanic activity came to an end. Hence, any solid carbonates were not recycled through volcanoes as they are on Earth. The depletion of carbon dioxide from the Martian atmosphere into the surface would therefore have been permanent.
As the amount of atmospheric CO2 declined, the greenhouse effect on Mars would have weakened and temperatures would have fallen. This temperature decrease would have caused more water vapor to condense into rain or snow and fall to the surface, taking even more CO2 with it and further weakening the greenhouse effect. Thus, a decrease in temperature would have caused a further decrease in temperature—a phenomenon sometimes called a runaway icehouse effect. (This is the reverse of the runaway greenhouse effect that has taken place on Venus.) Ultimately, both water vapor and most of the carbon dioxide would have been removed from the Martian atmosphere. With only a very thin CO2 atmosphere remaining, surface temperatures on Mars eventually would have stabilized at their present frigid values.
The atmosphere of Mars today, as Figure 6-18c shows, is very cold and thin compared to the atmosphere of either Earth or Venus—the surface pressure on Mars is a mere 0.006 atm. But its chemical composition is very close to that of Venus—the Martian atmosphere is 95.3% carbon dioxide (versus 96.5% for Venus) and 2.7% nitrogen (versus 3.5% for Venus). The predominance of carbon dioxide means that Mars, like Earth and Venus, is warmed by the greenhouse effect. However, the Martian atmosphere is so thin that the greenhouse effect is very weak and warms the Martian surface by only 40°F (versus 90°F on Earth). While the perpetual cloud cover on Venus maintains a steady surface temperature, the thin atmosphere on Mars provides very little thermal insulation. While daytime highs on Mars can be as high as 68°F (20°C), at night the temperature can plummet to −220°F (−140°C).
158
COSMIC CONNECTIONS Evolution of Terrestrial Atmospheres
Earth, Venus, and Mars formed with similar original atmospheres. However, these atmospheres changed dramatically over time due to factors such as planetary size and distance from the Sun.
159
Water vapor makes up about 0.03% of the Martian atmosphere (versus about 1% for Earth), and this vapor can form clouds. Figure 6-15b shows clouds that form near the tops of Martian volcanoes when air moves up the volcanic slopes. The rising air cools until the water vapor condenses into ice crystals. The same process occurs on Earth, but the clouds that form in this way are made of droplets of liquid water rather than ice crystals. Why is there a difference?
The explanation is that liquid water cannot exist anywhere on the Martian surface or in the Martian atmosphere. Water is liquid over only a limited temperature range: If the temperature is too low, water becomes ice, and if the temperature is too high, it becomes water vapor. What determines this temperature range is the atmospheric pressure above a body of water or around a water drop. If the pressure is very low, molecules easily escape from the liquid’s surface, causing the water to vaporize. Thus, at low pressures, water more easily becomes water vapor. The average surface temperature on Mars is only about −10°F (−23°C or 250 K), and the average pressure is only 0.006 atm. With this combination of temperature and pressure, water can exist as a solid (ice) and as a gas (water vapor) but not as a liquid. You can find a similar situation inside a typical kitchen freezer, where countless tiny water droplets swirl around over ice cubes looking like a fog. Hence, it never rains on Mars and there are no bodies of liquid water anywhere on the planet. In order to keep water on Mars in the liquid state, it would be necessary to increase both the temperature (to keep water from freezing) and the pressure (to keep the liquid water from evaporating).
At very high altitudes above the Martian surface, atmospheric carbon dioxide can freeze into crystals and form clouds. (Frozen carbon dioxide can be made on Earth and is called “dry ice.”) Such frozen carbon dioxide is also found in larger quantities in ice caps at the north and south poles of Mars, one of which you can see in Figure 6-1.
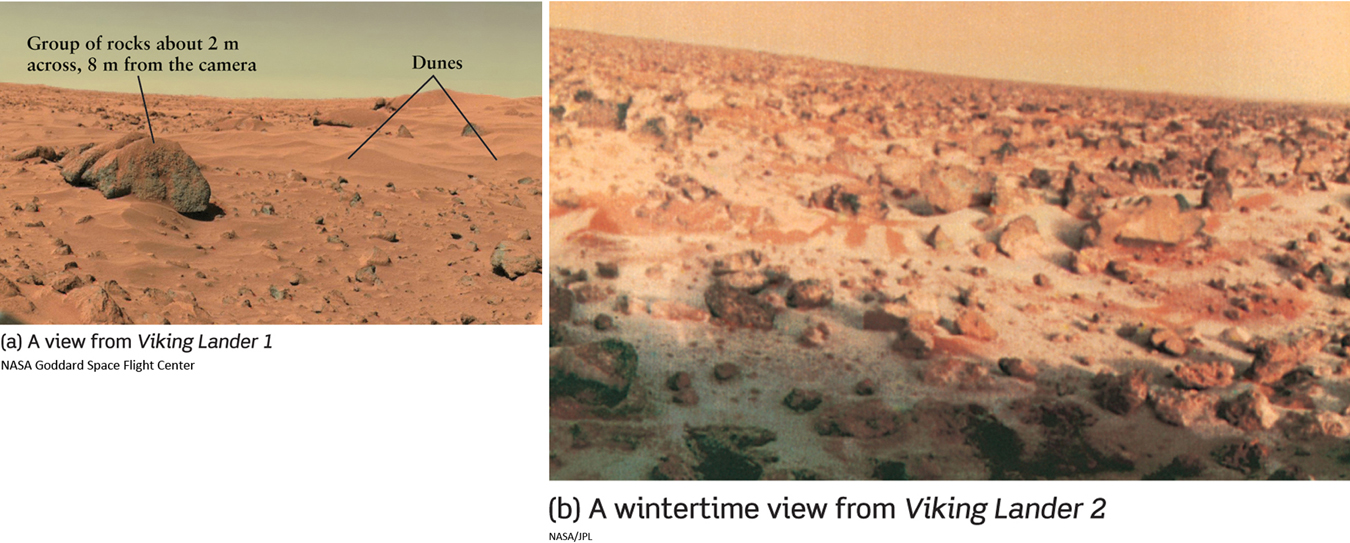
The wintertime freezing of atmospheric carbon dioxide has a curious effect. Figure 6-19a shows the springtime view from Viking Lander 1. Initially, the spacecraft measured pressures around 0.008 atm. After only a few weeks on the Martian surface, however, atmospheric pressure at both Viking sites was dropping steadily. As it turns out, when winter arrives, carbon dioxide freezes in that hemisphere’s atmosphere and falls as dry-ice “snow” (Figure 6-19b). The formation of this snow removes gas from the Martian atmosphere, thereby lowering the atmospheric pressure across the planet. Several months later, when spring comes, the dry-ice snow evaporates rapidly and the atmospheric pressure returns to its prewinter levels. Interestingly, both temperature and atmospheric pressure change significantly with the Martian seasons. Given these phenomena, which are quite different from that found on Earth, one wonders if the nature of atmospheres many times farther from the Sun are even more curious. As we will see in the next chapter, the largest planets have dynamic atmospheres driven by energy from both the distant Sun and energy sources within. But first, we will touch on a topic of great interest to scientists and many nonscientists as well—the search for water on other planets.
Question
ConceptCheck 6-11: Why might Mars’s environment become more hospitable to life if more CO2 could be added to the atmosphere?
Question
ConceptCheck 6-12: What would happen to water if an astronaut carelessly splashed some onto the Martian surface?