Chapter 1. The Nature of Light
Overview
Nature of Light and Matter
Astronomers are passionately interested in the nature of light because it is the only information we get from stars. The stars, and most of the planets for that matter, are so far away that we will probably never visit them. So, for now, astronomers must learn to decode information hidden in starlight if we are to learn anything at all about the nature of these distant objects. Fortunately, the properties of light are well understood. After starlight is split into a rainbow by passing it through a prism or similar device, careful inspection of the light provides detailed information about the temperature, composition, and motion of stars.

Observation
Nature of Light and Matter

When sunlight passes through a prism, it is spread out into all of its colors. Using a ruler, carefully measure the width in millimeters, of each color in the rainbow shown. In the space, list each color in the rainbow in order from largest to smallest width.
Tutorials
(In the original, there is no action taken when "Tutorials" heading is clicked, so no text would appear immediately below the "Tutorials" heading here either.)
Light and Temperature (first tutorial/L2 section)
Considering Star Colors and Temperatures
When looking up at the night sky, you might notice that stars appear in a variety of brightnesses and colors. Five bright stars and their temperatures are listed below. Estimate the color each would appear to be in the sky and explain your reasoning.
1. Aldebaran (approximate temperature 4000 K)
2. Pollux (approximate temperature 4200 K)
3. Sun (approximate temperature 5800 K)
4. Procyon (approximate temperature 6700 K)
5. Altair (approximate temperature 7000 K)
You are not required to provide the correct answer to get full credit. But you must give a detailed and thoughtful response, which will be shown to you again at the end of this tutorial. When you send your response to the course instructor, you will receive credit for a thoughtful response.
Nature of Light and Electromagnetic Radiation
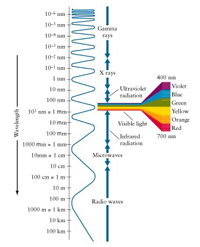
Although we use light every day, many of us have not stopped to think about the nature of light. What are the main characteristics of light, anyway? Light is composed of tiny packets of energy called photons. Light moves extremely fast—nearly 300,000 kilometers every second (186,000 miles per second). Light is often described as having wave-like characteristics called wavelengths. A more appropriate name for light and light energy is electromagnetic radiation or EM radiation. The EM radiation our eyes are capable of seeing is usually called visible light and ranges in wavelengths from about 400 to 700 nanometers (A nanometer is one-billionth of a meter). Stars and most other objects, as it turns out, emit a wide range of different wavelengths of EM radiation in addition to visible light. Most of the names for EM radiation are familiar to you. Listed from short wavelengths to long wavelengths, there are gamma rays, X rays, ultraviolet light, visible light, infrared light, microwaves, and radio waves. In fact, visible light constitutes only a minuscule portion of the types of EM radiation emitted by stars. Because each type of EM radiation has a different wavelength, each also has a different energy. Energy and wavelength are inversely proportional. Blue light, for instance, has a much higher energy and a much shorter wavelength than red light, and red light has much lower energy but much longer wavelength than blue light.
Self Test: Electromagnetic Spectrum

Drag the circles adjacent to the terms below to their appropriate location on the diagram.
Click here (http://bcs.whfreeman.com/aol/content/cat_030/ch05/m05t001p3ST.html) to view the exercise in an external window.
Emission of Light by Electrons in Atoms
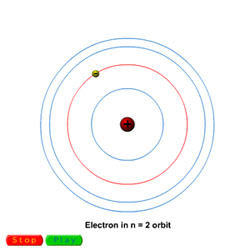
Light is emitted by electrons moving from higher energy states in an atom to lower energy states
When atoms are heated or bathed in EM radiation, electrons absorb the extra energy and move to higher states when orbiting the nucleus. Because electrons are negatively charged and the particles in the nucleus are positively charged, the electrons quickly return to their lower orbits. However, to move to a lower orbit, the electron must release some energy. Electrons release this energy in the form of light.
Temperature and Color
When atoms in objects absorb and reemit higher and higher levels of energy, such as when they are heated to thousands of degrees, they emit shorter and shorter wavelengths of light. This simple, inversely proportional relationship between the temperature, measured in kelvins, of an emitting object and the maximum wavelength of light emitted, , measured in meters, is known as Wien's law. Wien's law is calculated as
max meters= 0.0029 / T kelvin
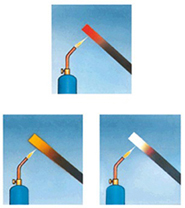
What this means is that the hotter an object is, the shorter the wavelength, or bluer, the light being emitted is. Objects that are at tens of thousands of degrees emit mostly ultraviolet light. Gases, like those in the outer atmosphere of the Sun, are at millions of degrees and emit primarily X rays.
Self Test: Wien's Law

According to Wien's law, the wavelength of maximum emission of a blackbody is inversely proportional to its temperature in Kelvins. Given the maximum wavelength of light emitted by a star in nanometers, use the on-line Wien's law calculator below to determine the approximate surface temperature of the following stars to rank order them from hottest to coolest. Note: 206 nm is equal to 206 X 10-9 m.
Rigel, max = 206 nm
Deneb, max = 302 nm
Arcturus, max = 644 nm
Vega, max = 251 nm
Betelgeuse, max = 935 nm
To use this calculator enter the observed peak wavelength (lambdamax). Press the OK button to determine the object's temperature in kelvin (K). Note: "e" stands for a power-of-ten exponent. For example, 5.8e3 = 5.8 x 103, 5e-7 = 5.0 x 10-7.
Infrared Radiation
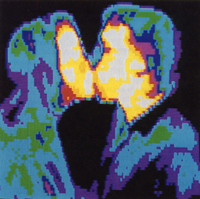
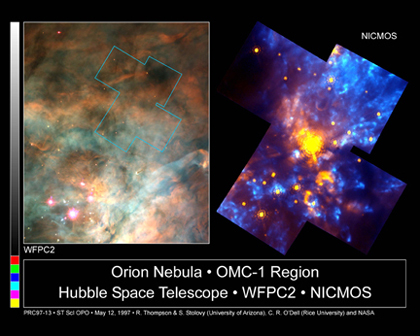
Because our eyes are sensitive to only a very small range of EM radiation, we often do not realize that all objects emit EM radiation. In fact, all objects that have a temperature above absolute zero (-273°C) emit some EM radiation. Objects like your computer mouse are at room temperature, or about 295 K. The computer mouse is emitting some EM radiation right this instant, most likely in the infrared, which our eyes do not detect. The reason you can see your computer mouse is that it is reflecting visible light in the room into your eyes. Infrared radiation is an important part of astronomy because infrared radiation can come through the interstellar clouds, which visible light does not penetrate. In the Hubble Space Telescope photograph, the left side shows only a few visible stars whereas the camera that is sensitive to infrared light can easily see the infrared radiation from the cool stars within the clouds.
Note: The Jet Propulsion Laboratory has created a short video about infrared radiation that is available online at http://coolcosmos.ipac.caltech.edu/
Blackbody Radiation Curves
Complete all questions first, then click "Save answer" below, before you leave this page.
All objects emit a wide range of EM radiations. We can make a graph of the emitted radiations of any object by plotting the wavelength emitted versus the brightness. The resulting curve is called a blackbody curve. A blackbody curve illustrates the intensity of light at every wavelength that is emitted by a blackbody (an idealized case of energy emission from a hot and dense object) at a particular temperature. The higher the temperature, the shorter the wavelength of maximum emission (at which the curve peaks) and the greater the amount of light emitted at every wavelength.
INSTRUCTIONS: To graph a blackbody curve, enter the temperature (in kelvins) and click on "New Curve". Up to five curves can be displayed at one time. The "Clear" button will remove all curves. The "Redraw" button will refresh the graph. This is useful when the function domain (i.e., wavelength) has been changed. To see the temperature for each curve, check the "Temperature" box. Check the "Wien's law" box to see the wavelength of maximum emission. Check the "Visible light", "Ultraviolet", or "Infrared" boxes to delineate that portion of the spectrum.
Spectroscopy (second tutorial/L2 section)
......subsections of content go here.
Activities
Introductory text for activities.
...Light and Temperature (L2 section)
......Java Wavelength Calculator app and questions go here.
...Spectroscopy (L2 section)
......Java Doppler app and questions go here.
Examination
Introductory text for 20 questions that follow.
1.
New Paragraph
Question text
| A. |
| B. |
| C. |