Chapter 1. Hayden-McNeil
Experiment 23
Voltaic and Electrolytic Cells
Prelab Assignment
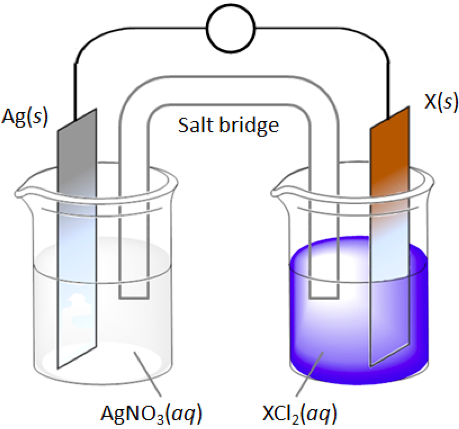
The voltaic cell above is constructed using Ag(s) on one side and one of several randomly selected metals on the other. The reduction half reaction of each of the metals is as follows:
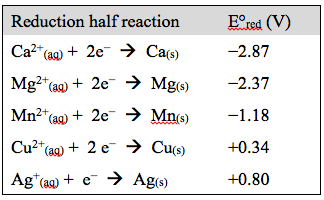
Your randomly selected metal is Mn. Answer the following questions:
1.1 Question 1
1.
At which reaction will oxidation occur?
| A. |
| B. |
At which reaction will reduction occur?
| A. |
| B. |
Which strip of metal acts as the anode?
| A. |
| B. |
What half-reaction occurs at the anode?
| A. |
| B. |
| C. |
| D. |
Which strip of metal acts as the cathode?
| A. |
| B. |
What half-reaction occurs at the cathode?
| A. |
| B. |
| C. |
| D. |
| E. |
Which strip of metal gains mass as the reaction proceeds?
| A. |
| B. |
In which direction do the electrons flow?
| A. |
| B. |
Assuming both solutions are 1.0 M in concentration and T = 298 K, what voltage will you read at the multimeter?
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
1.2 Question 2
Sodium metal is produced by electrolysis of molten NaCl. Answer the following questions for this process.
2.
At which electrode is metallic sodium produced?
| A. |
| B. |
3.
What is produced at the other electrode?
| A. |
| B. |
| C. |
| D. |
| E. |
4.
How long would you have to pass a current of x A to produce 85 g of sodium metal?
| A. |
| B. |
| C. |
| D. |
| E. |
Activity results are being submitted...