Chapter 1. Hayden-McNeil
Experiment 23
Voltaic and Electrolytic Cells
Prelab Assignment
1.1 Question 1
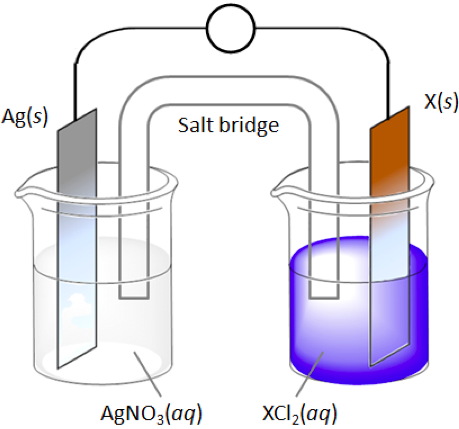
The students are given the diagram shown above. The unknown metal X will randomly be shuffled to be one of the following X = Cu or Mn or Mg or Ca. They would also be shown the following table.
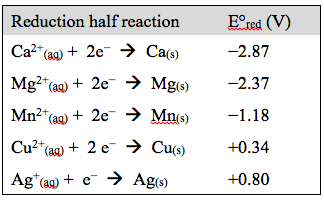
With this information available they would be asked to answer the following questions:
1.
At which reaction will oxidation occur?
| A. |
| B. |
2.
At which reaction will reduction occur?
| A. |
| B. |
3.
Which strip of metal acts as the anode?
| A. |
| B. |
4.
What half-reaction occurs at the anode?
| A. |
| B. |
| C. |
| D. |
| E. |
5.
Which strip of metal acts as the cathode?
| A. |
| B. |
6.
What half-reaction occurs at the cathode?
| A. |
| B. |
| C. |
| D. |
| E. |
7.
Which strip of metal gains mass as the reaction proceeds?
| A. |
| B. |
8.
In which direction do the electrons flow?
| A. |
| B. |
9.
Assuming both solutions are 1.0 M in concentration and T = 298 K, what voltage will you read at the multimeter?
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
1.2 Question 2
Sodium metal is produced by electrolysis of molten NaCl. Answer the following questions for this process.
10.
At which electrode is metallic sodium produced?
| A. |
| B. |
11.
What is produced at the other electrode?
| A. |
| B. |
| C. |
| D. |
| E. |
12.
How long would you have to pass a current of x A to produce 85 g of sodium metal?
| A. |
| B. |
| C. |
| D. |
| E. |
Activity results are being submitted...