Chapter 1. Week 2 Experiment
Purpose

To separate the components of a mixture and determine the mass percent of each present.
Discussion
Separation of the components of a mixture is a common problem in a chemistry laboratory. If we want to study the properties of a substance, it must fi rst be isolated, that is, separated from other substances with which it occurs naturally. If we prepare a product in the laboratory, it is often mixed with other products or excess reactants.
Separation of a mixture is a physical process that depends on a difference in a physical property of the components. An example is the separation of bone fragments from clay and sand at an archeological dig. Bone is less dense than sand and clay; the separation is based on this difference in density. The mixture of bone, sand and clay is added to a solution that has a density greater than bone but less than sand and clay. The bone fragments fl oat and the sand and clay fall to the bottom.
Copper is sometimes found in nature as the free metal. The copper-containing ore is heated to a temperature just above the melting point of copper. The liquid copper can then be poured off, separating it from higher-melting substances.
In this experiment, we are separating the components of a mixture by using differences in solubility. Solubility is the amount of a substance, the solute, that will dissolve in a given amount of another substance, the solvent. Solubility is greater when solute and solvent are more alike. Organic compounds contain carbon and a few other elements such as hydrogen and oxygen. Organic compounds tend to be soluble in organic solvents. Inorganic compounds (which contain elements other than carbon) tend to be soluble in water and insoluble in organic solvents. Some compounds, such as inorganic oxides and carbonates, are insoluble in both water and organic solvents.
Your sample is a mixture that may contain an organic component, a water-soluble inorganic component, and a metal carbonate. The mixture can be separated by using differences in solubilities.
The procedure has four parts:
- Weigh the sample and containers.
- Extract the sample with organic solvent to remove the organic component, X.
- Extract the sample with water to remove the water-soluble inorganic component, Y.
- Weigh the remaining insoluble component, Z.
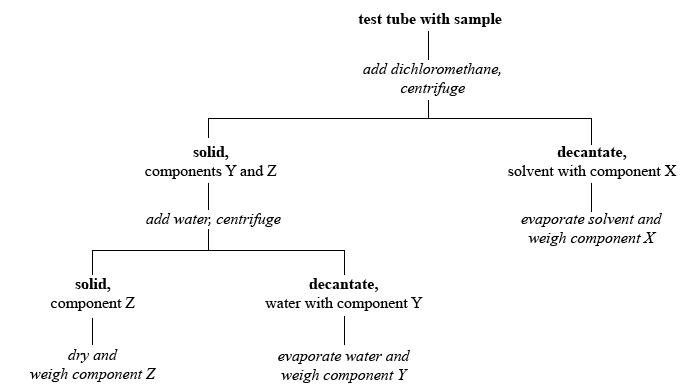
Addition of the organic solvent dichloromethane to the mixture will dissolve the organic compound. The process of dissolving a compound out of a mixture is called extraction. The solvent containing the soluble component—in this case organic compound X—must then be removed from remaining solid. On a larger scale, this would be accomplished by filtration. On a semimicro scale—quantities that we are using—the separation is achieved by centrifuging and decanting. A centrifuge is a mechanical device that rotates test tubes causing the solid, more dense, component of the mixture to quickly gravitate to the bottom of the test tube. Decanting is the process of pouring off the liquid and leaving solid undisturbed, thereby achieving separation. Instead of pouring, we will remove the liquid using a micropipet (a drawn-out medicine dropper).
After the organic compound is removed, the extraction process is repeated using water as the solvent. The water-soluble inorganic component Y is removed in this process, leaving the water-insoluble component Z.
The following is a flowchart that summarizes the procedure.
Prelaboratory Questions
Use the CRC Handbook of Chemistry and Physics (available in the Science and Engineering Library) to find solubility information for compounds listed below. Information will be found in either the table of “Physical Constants of Inorganic Compounds” or “Physical Constants of Organic Compounds.”
| Compound | Organic or Inorganic | Solubility In Water | Solubility in Other Solvents | Classify as X,Y,X |
|---|---|---|---|---|
| barium chloride, BaCl2 | ||||
| iron(ll) carbonate, FeCO2 | ||||
| lead(ll) oxide, PbO | ||||
| strychnine, C21H22N2O2 | ||||
| tin(II) sulfate, SnSO4 | ||||
| vitamin E or α-tocopherol, C29H50O2 |
Based on your tabulated differences in solubilities, choose a solvent that would separate each of the following pairs. State which compound would dissolve and which would remain solid.
and
and strychnine
and vitamin E
PbO and
FB: PbO and strychnine
and vitamin E
A solid sample containing a mixture of BaCl2, PbO, and vitamin E was treated according to the procedure in this experiment. Suggest a solvent to be used for part B and identify the solids as component X, Y or Z.
A solid sample containing a mixture of FeCO3, strychnine, and SnSO4 was treated according to the procedure in this experiment. Suggest a solvent to be used for part B and identify the solids as component X, Y or Z.
There is an online prelaboratory problem for this experiment which is accessed via this link: http://undergrad-ed.chemistry.ohio-state.edu/genchem/REPORT
Activity Completed!
Activity results are being submitted...