Chapter 1. Pre-lab Experiment 3: Chemical Equation
Introduction
Welctome to PreLab Experiment 3 Quiz
To Begin your quiz, click the Next button.
1.1 Question 1
Which of the following must be the same before and after a chemical reaction?
| A. |
| B. |
| C. |
| D. |
| E. |
Question 2
Identify the reaction type for the following:
2 KClO3(s) → 2 KCl(s) + 3 O2(g)
| A. |
| B. |
| C. |
| D. |
Identify the reaction type for the following:
MgO(s) + CO2 (g) → MgCO3 (s)
| A. |
| B. |
| C. |
| D. |
Identify the reaction type for the following:
NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s)
| A. |
| B. |
| C. |
| D. |
Identify the reaction type for the following:
C10H8 (s) + 12O2 (g) → 10 CO2 (g) + 4 H2O (l)
| A. |
| B. |
| C. |
| D. |
Question 3
The reaction between reactant A (smaller spheres) and reactant B (larger spheres) is shown in the diagram.
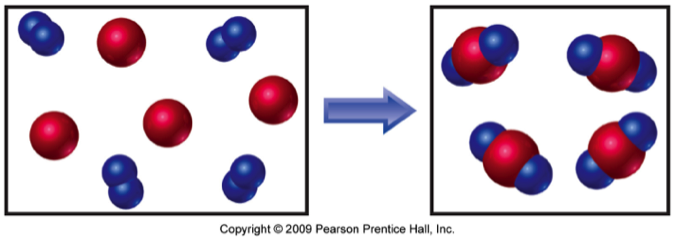
Based on this diagram, which equation best describes the reaction?
| A. |
| B. |
| C. |
| D. |
The reaction between reactant A (smaller spheres) and reactant B (larger spheres) is shown in the diagram.
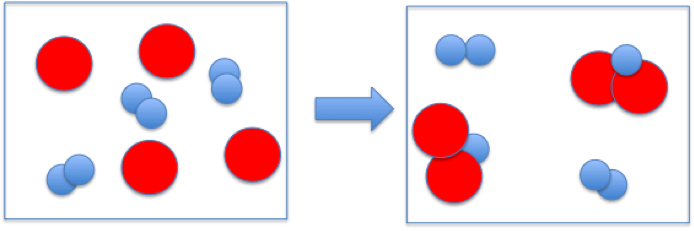
Based on this diagram, which equation best describes the reaction?
| A. |
| B. |
| C. |
| D. |
The reaction between reactant A (smaller spheres) and reactant B (larger spheres) is shown in the diagram.
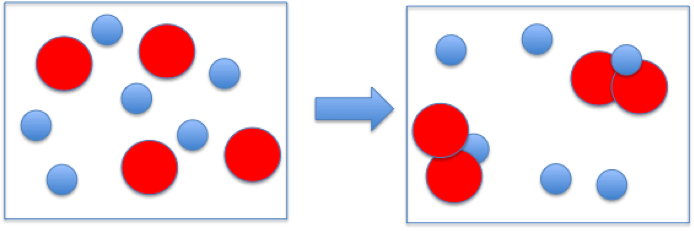
Based on this diagram, which equation best describes the reaction?
| A. |
| B. |
| C. |
| D. |
The reaction between reactant A (smaller spheres) and reactant B (larger spheres) is shown in the diagram.
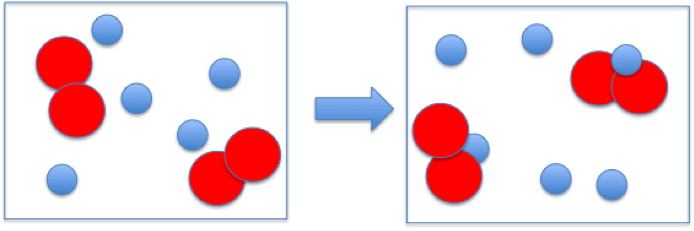
Based on this diagram, which equation best describes the reaction?
| A. |
| B. |
| C. |
| D. |
Question 4
Four drops of solution “A” are combined with eight drops of solution “B” and a precipitate forms. The supernatant is then tested by combining it with solution “C” and the precipitate “AC” forms. Which inference is consistent with this observation?
| A. |
| B. |
| C. |
| D. |
Four drops of solution “A” are combined with eight drops of solution “B” and a precipitate forms. The supernatant is then tested by combining it with solution “C” and the precipitate “BC” forms. Which inference is consistent with this observation?
| A. |
| B. |
| C. |
| D. |
Question 5
H2(g) and O2(g) are being combined to produce H2O (g). When will the hydrogen gas be in excess?
| A. |
| B. |
| C. |
| D. |
| E. |
H2(g) and O2(g) are being combined to produce H2O (g). When will the hydrogen gas be the limiting reactant?
| A. |
| B. |
| C. |
| D. |
| E. |
H2(g) and O2(g) are being combined to produce H2O (g). When will the oxygen gas be the limiting reactant?
| A. |
| B. |
| C. |
| D. |
| E. |
Activity results are being submitted...