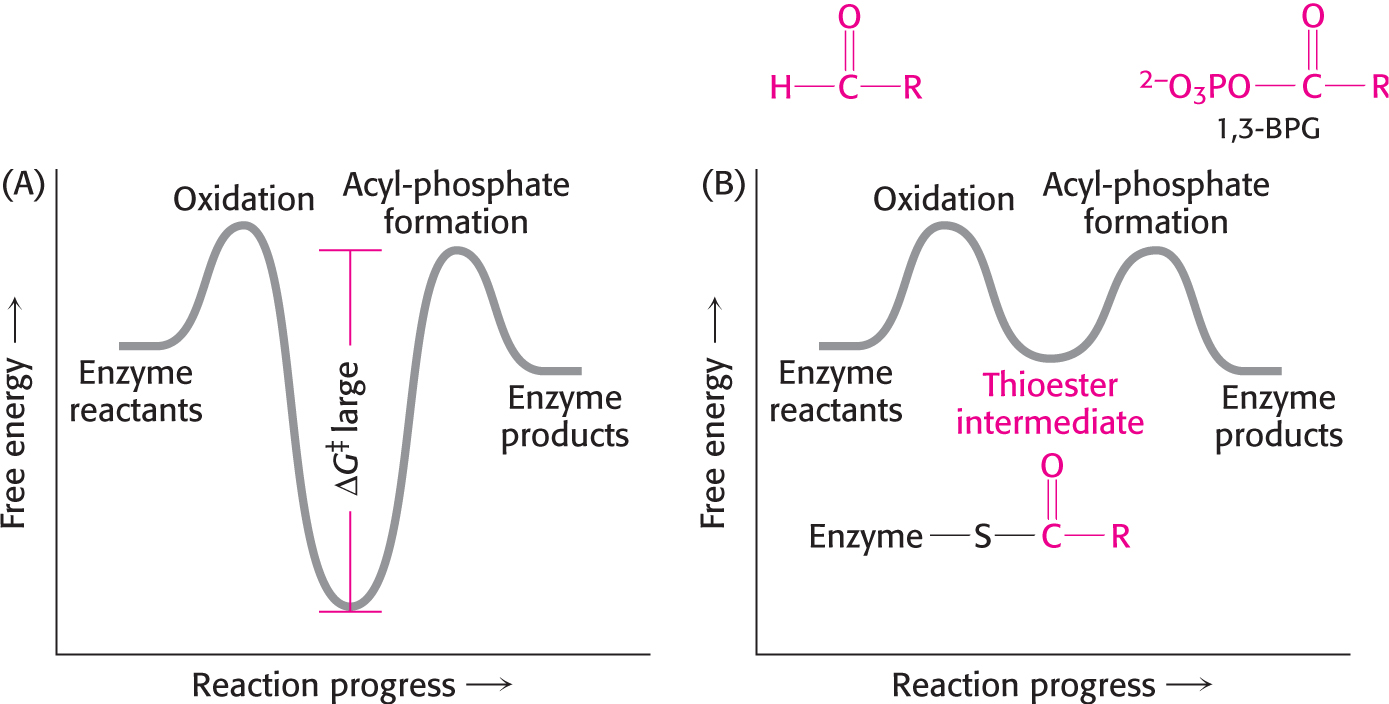
Figure 16.3 Free-energy profiles for glyceraldehyde oxidation followed by acyl-phosphate formation . (A) A hypothetical case with no coupling between the two processes. The second step must have a large activation barrier, making the reaction very slow. (B) The actual case with the two reactions coupled through a thioester intermediate. The thioester intermediate is more stable than the reactant, and, hence, its formation is spontaneous. However, the intermediate is less stable than the product, which forms spontaneously. Thus, the barrier separating oxidation from acyl-phosphate formation is eliminated.
[Leave] [Close]