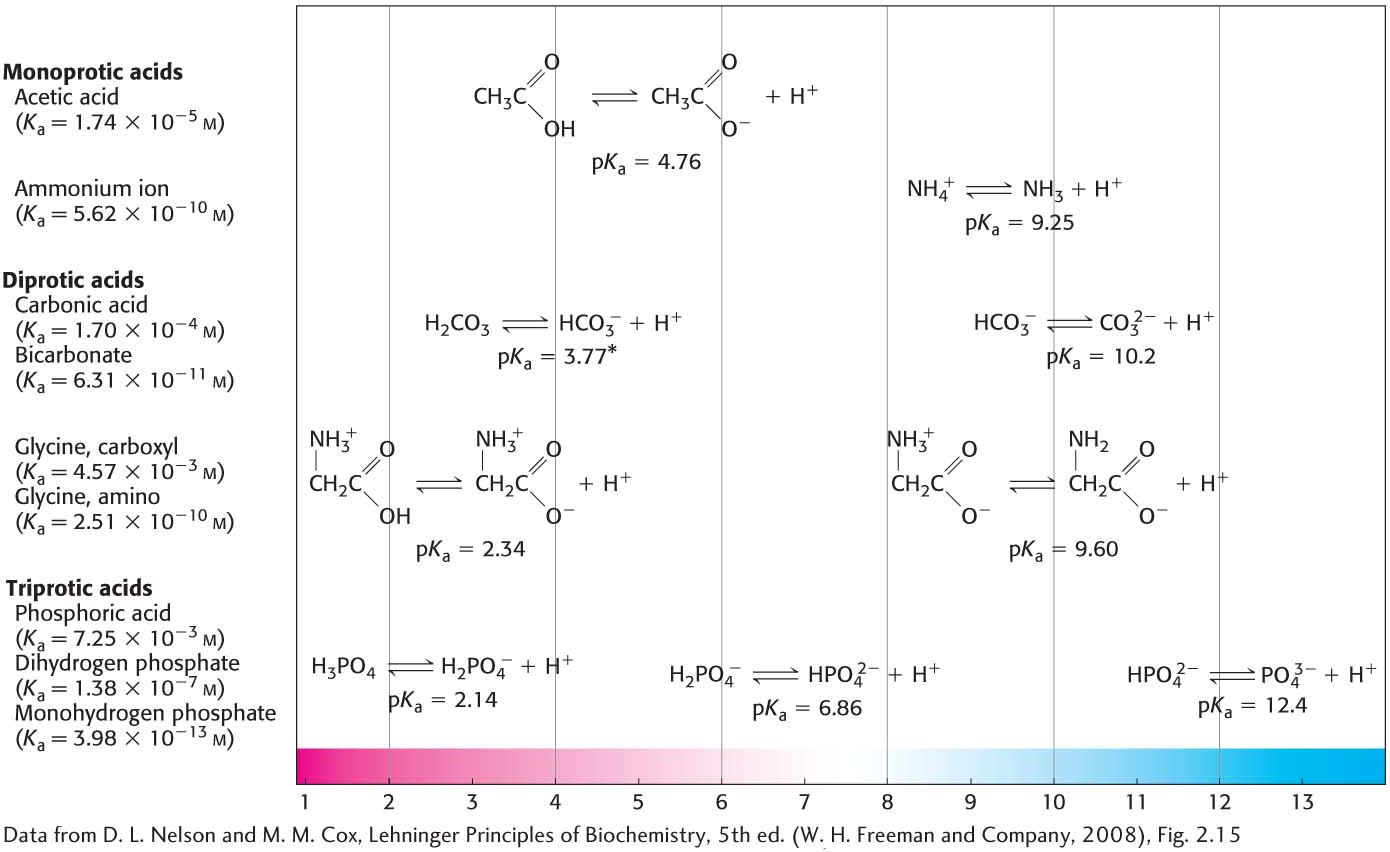
Figure 2.11 A variety of conjugate acid–base pairs . Many conjugate acid–base pairs are important in biochemistry. A sampling of such pairs is shown. Notice that some acids can release more than one proton. The dissociation reaction and the pKa for each pair are shown where they lie along a pH gradient. The equilibrium constants for the acids are given at the left of the illustration. *Note the pKa of carbonic acid. Because of the large reservoir of carbon dioxide in the blood and its rapid equilibration with carbonic acid, the pKa of carbonic acid in blood is taken to be 6.1.
[Leave] [Close]