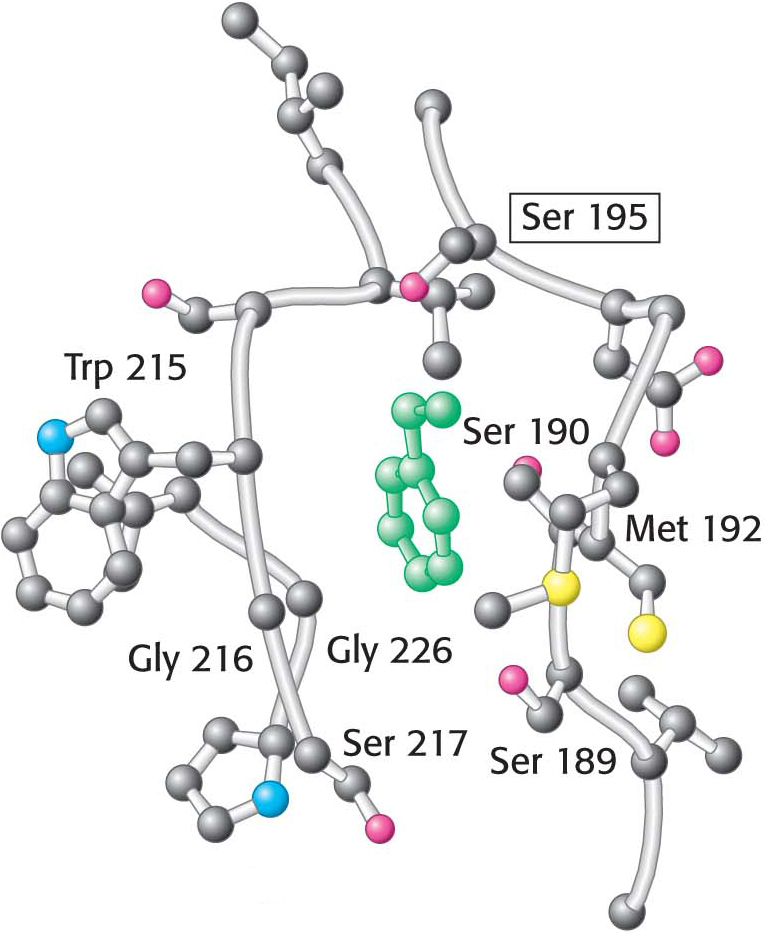
Figure 8.27 The specificity pocket of chymotrypsin. Notice that many hydrophobic groups line the deep specificity pocket. The structure of the pocket favors the binding of residues with long hydrophobic side chains such as phenylalanine (shown in green). Also notice that the active-site serine residue (serine 195) is positioned to cleave the peptide backbone between the residue bound in the pocket and the next residue in the sequence. The key amino acids that constitute the binding site are identified.
[Leave] [Close]