Chapter 11
Lipids are water-
insoluble molecules that are highly soluble in organic solvents. Complete the interactive matching exercise to see answers.
Triacylglycerols from plants may have many cis double bonds or have shorter fatty acid chains than those from animals.
Triacylglycerols consist of three fatty acid chains attached to a glycerol backbone. Triacylglycerols are a storage form of fuel. Phosphoglycerides consist of two fatty acid chains attached to a glycerol backbone. The remaining alcohol of the glycerol is bonded to a phosphate, which is in turn bonded to an alcohol. Phosphoglycerides are membrane components.
The backbone in phosphoglycerides is glycerol, whereas that in sphingolipids is sphingosine. In sphingolipids, one of the fatty acids is linked to the sphingosine by an amide bond.
Examples of head groups include serine, ethanolamine, choline, glycerol, and inositol.
 Page C12
Page C12Lipids are primarily hydrophobic molecules. For instance, triacylglycerols have three fatty acid side chains. This predominately hydrophobic nature accounts for their solubility in organic solvents and their lack of solubility in aqueous solvents.
The hydrophobic chains would shun the water, interacting with similar chains in other molecules. Meanwhile, the hydrophilic head groups would readily interact with the water, resulting in the formation of a membrane or a small membrane vesicle called a liposome.
Steroids are cyclical rather than linear.
The C16 fatty acid is attached by an ether linkage. The C-
2 carbon atom of glycerol has only an acetyl group attached by an ester linkage instead of a fatty acid, as is the case with most phospholipids. 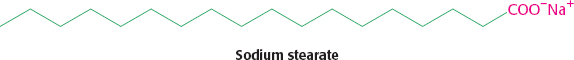
The sodium stearate will form a micelle, with the hydrophilic head groups (red) exposed to water and the fatty acid chains (green) in the interior. When the sodium stearate is worked into the clothes by agitation or onto the skin by rubbing in the presence of water, the grease, which is hydrophobic, will localize in the hydrophobic interior of the micelle and be washed down the drain with rinsing.
Instead of forming a soluble micelle that will be washed down the drain, the magnesium or calcium salts will precipitate, forming a scum-
like bathtub ring. You should clean the bathtub immediately because, when the scum dries, it is more difficult to remove. Lipids are more reduced than glycogen, and they are stored in an anhydrous form.
Hibernators selectively feed on plants that have a high proportion of polyunsaturated fatty acids with lower melting temperature.
Complete the interactive matching exercise to see answers.
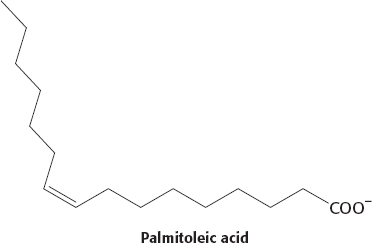
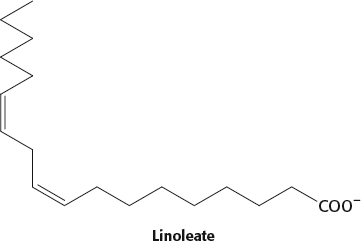
The presence of a cis double bond introduces a kink in the fatty acid chain that prevents tight packing and reduces the number of atoms in van der Waals contact. The kink will lower the melting point compared with that of a saturated fatty acid. Trans fatty acids do not have the kink, and so their melting temperatures will be higher, more similar to those of saturated fatty acids.
Palmitic acid is shorter than stearic acid. Thus, when the chains pack together, there will be less opportunity for van der Waals interactions and the melting point will thus be lower than that of the longer stearic acid.