Chapter 19
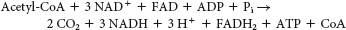
The citric acid cycle depends on a steady supply of NAD+ and FAD, which are typically generated from NADH and FADH2 by reaction of these electron carriers with oxygen. If there is no oxygen to accept the electrons, the citric acid cycle will cease to operate.
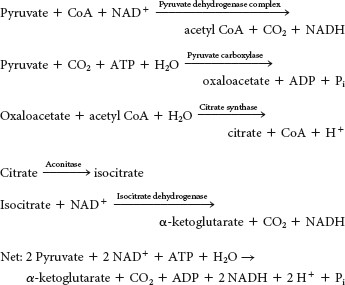
In the first stage, two carbon atoms are introduced into the cycle by reacting with oxaloacetate to form citrate. Two carbon atoms are then oxidized to CO2. In the second stage, the resulting four-
carbon molecule is metabolized to regenerate oxaloacetate. High- energy electrons are generated in both stages. Complete the interactive matching exercise to see answers.
The reaction is powered by the hydrolysis of a thioester. Acetyl CoA provides the thioester that is converted into citryl CoA. When this thioester is hydrolyzed, citrate is formed in an irreversible reaction.
Acetyl CoA does not bind citrate synthase until oxaloacetate is bound and ready for the synthesis of citrate. In addition, the catalytic groups required for the hydrolysis of the thioester are not positioned for catalysis until citryl CoA has formed.
Isocitrate lyase and malate synthase are required in addition to the enzymes of the citric acid cycle.
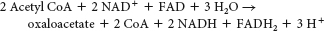
No. Hence, mammals cannot carry out the net synthesis of oxaloacetate from acetyl CoA.
−41.0 kJ mol−1 (−9.8 kcal mol−1)
Enzymes or enzyme complexes are biological catalysts. Recall that a catalyst facilitates a chemical reaction without the catalyst itself being permanently altered. The citric acid cycle operates catalytically in that oxaloacetate binds to an acetyl group, leads to the oxidative decarboxylation of the two carbon atoms, and is regenerated at the completion of a cycle.
Succinate will increase in concentration, followed by α-ketoglutarate and the other intermediates “upstream” of the site of inhibition. Succinate has two methylene groups that are required for the dehydrogenation, whereas malonate has but one.
Succinate dehydrogenase is the only enzyme in the citric acid cycle that is embedded in the mitochondrial membrane in association with the electron-
transport chain. The reaction catalyzed by succinyl CoA synthetase:
Succinyl CoA + Pi + ADP → succinate + CoA + ATP
The reaction catalyzed succinyl CoA synthetase:
Succinyl CoA + Pi + ADP → succinate + CoA + ATP
Isocitrate dehydrogenase and α-ketoglutarate dehydrogenase
It enables organisms such as plants and bacteria to convert fats, through acetyl CoA, into glucose.
Both α-ketoglutarate and pyruvate are α-ketoacids that are decarboxylated to form thioesters of CoA. Moreover, the dihydrolipoyl dehydrogenase components of the complexes are identical and the other enzymes are similar. Finally, the reaction mechanisms are the same.
We cannot attain the net conversion of fats into glucose, because the only means to get the carbon atoms from fats into oxaloacetate, the precursor to glucose, is through the citric acid cycle. However, although two carbon atoms enter the cycle as acetyl CoA, two carbon atoms are lost as CO2 before oxaloacetate is formed. Thus, although some carbon atoms from fats may end up as carbon atoms in glucose, we cannot obtain a net synthesis of glucose from fats.
Pyruvate carboxylase should be active only when the acetyl CoA concentration is high. Acetyl CoA might accumulate if the energy needs of the cell are not being met, because of a deficiency of oxaloacetate. Under these conditions the pyruvate carboxylase catalyzes an anapleurotic reaction. Alternatively, acetyl CoA might accumulate because the energy needs of the cell have been met. In this circumstance, pyruvate will be converted back into glucose, and the first step in this conversion is the formation of oxaloacetate.
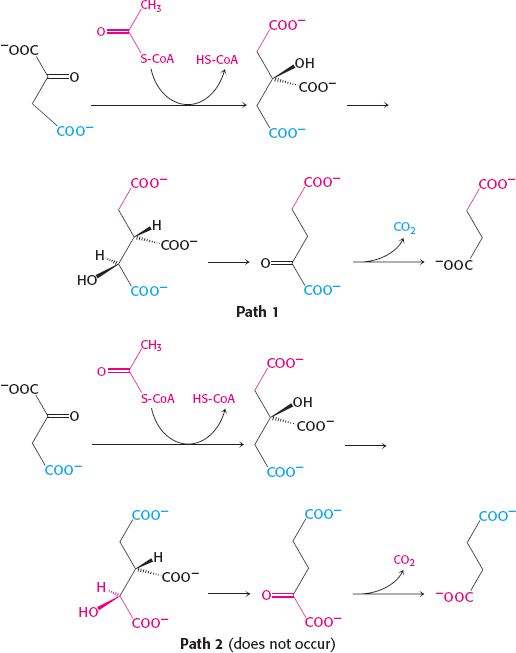
Citrate is a symmetric molecule. Consequently, the investigators assumed that the two −CH2COO− groups in it would react identically. Thus, for every citrate molecule undergoing the reactions shown in path 1, they thought that another citrate molecule would react as shown in path 2. If so, then only half the label should have emerged in the CO2.
 Page C20
Page C20Call one hydrogen atom A and the other B. Now suppose that an enzyme binds three groups of this substrate—
X, Y, and H— at three complementary sites. The adjoining diagram shows X, Y, and HA bound to three points on the enzyme. In contrast, X, Y, and HB cannot be bound to this active site; two of these three groups can be bound, but not all three. Thus, HA and HB will have different fates. 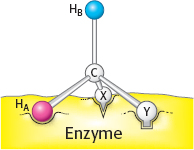
Sterically nonequivalent groups such as HA and HB will almost always be distinguished in enzymatic reactions. The essence of the differentiation of these groups is that the enzyme holds the substrate in a specific orientation. Attachment at three points, as depicted in the diagram, is a readily visualized way of achieving a particular orientation of the substrate, but it is not the only means of doing so.
The complete oxidation of citrate requires 4.5 µmol of O2 for every micromole of citrate:
C6H8O7 + 4.5 O2 → 6 CO2 + 4 H2O
Thus, 13.5 µmol of O2 would be consumed by 3 µmol of citrate.
Citrate led to the consumption of far more O2 than can be accounted for simply by the oxidation of citrate itself. Citrate thus facilitated O2 consumption.
In the absence of arsenite, the amount of citrate remained constant. In its presence, the concentration of citrate fell, suggesting that it was being metabolized.
Arsenite’s action is not altered. Citrate still disappears.
Arsenite is preventing the regeneration of citrate. Recall that arsenite inhibits the pyruvate dehydrogenase complex.
The initial infection is unaffected by the absence of isocitrate lyase, but the absence of this enzyme inhibits the latent phase of the infection.
Yes
A critic could say that, in the process of deleting the isocitrate lyase gene, some other gene was damaged, and the absence of this other gene prevents latent infection. Reinsertion of the isocitrate lyase gene into the bacteria from which it had been removed renders the criticism less valid.
Isocitrate lyase enables the bacteria to synthesize carbohydrates that are necessary for survival, including carbohydrate components of the cell membrane.
(a) After one round of the citric acid cycle, the label emerges in C-
2 and C- 3 of oxaloacetate. (b) The label emerges in CO2 in the formation of acetyl CoA from pyruvate. (c) After one round of the citric acid cycle, the label emerges in C- 1 and C- 4 of oxaloacetate. (d and e) Same fate as that in part a. (a) The steady-
state concentrations of the products are low compared with those of the substrates. (b) The ratio of malate to oxaloacetate must be greater than 1.57 × 104 for oxaloacetate to be formed. The energy released when succinate is reduced to fumarate is not sufficient to power the synthesis of NADH but is sufficient to reduce FAD.
Citrate is a tertiary alcohol that cannot be oxidized, because oxidation requires a hydrogen atom to be removed from the alcohol and a hydrogen atom to be removed from the carbon atom bonded to the alcohol. No such hydrogen exists in citrate. The isomerization converts the tertiary alcohol into isocitrate, which is a secondary alcohol that can be oxidized.