Chapter 2
Brownian motion is the random movement of molecules in a fluid or gas powered by the background thermal energy.
Water is polar in that the hydrogen atoms bear a partial positive charge, whereas the oxygen atom has a partial negative charge owing to the electronegative nature of the oxygen atom. However, the total charge on the molecule is zero; that is, the positive charges are equal to the negative charges.
Many weak bonds allow for highly specific yet transient interactions.
Ionic bonds, hydrogen bonds, and van der Waals interactions. Water disrupts ionic bonds and hydrogen bonds. Because van der Waals interactions are most common between hydrophobic groups, water can be said to strengthen these bonds by facilitating their formation through the hydrophobic effect.
Lowering the temperature would reduce the motion of the water molecules and allow the formation of more hydrogen bonds, which is indeed the case: each molecule of water in ice is hydrogen bonded to approximately 3.7 molecules of water. The opposite takes place as the water is heated, and fewer hydrogen bonds would be expected to form. At 100°C, a molecule of water is hydrogen bonded to 3.2 water molecules.
Electrostatic interactions would be stronger in an organic solvent relative to a polar solvent because there would be no competition from the solvent for the components of the electrostatic interaction.
An electronegative atom is one that has a high affinity for electrons. Consequently, when bonded to a hydrogen atom, the electronegative atom assumes a partial negative charge and the hydrogen atom assumes a partial positive charge. Such polarity allows the formation of hydrogen bonds.
The hydrophobic effect is the tendency of nonpolar molecules to interact with one another in the presence of water. The interaction is powered by the increase in entropy of water molecules when the nonpolar molecules are removed from the watery environment.
The Second Law of Thermodynamics states that the entropy of a system and its surroundings always increases in a spontaneous process. When hydrophobic molecules are sequestered away from water, the entropy of the water increases. Such sequestration, called the hydrophobic effect, also leads to the formation of biochemical structures.
10−9 M
10−12 M
The Henderson–
Hasselbalch equation is pH = pKa + log[A−]/[HA]. If [A−] = [HA], then the equation becomes pH = pKa + log 1. But log 1 = 0. Thus, pH = pKa under the conditions stated. The lower the pKa, the greater the Ka. The greater the Ka, the stronger the acid.
Recall that pKa = log 1/Ka. The antilog of 4.76, the pKa of acetic acid, is 57,543, and the Ka is the inverse. Thus, the Ka for acetic acid = 1.7 × 10−5.
The pKa for trichloroacetic acid is 0.7. Calculating as above, the Ka is 2.0 × 10−1.
Trichloroacetic acid is a stronger acid; that is, it is more completely dissociated.
As with many problems in life, this one can be solved with the Henderson–
Hasselbalch equation: 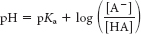
Pyruvic acid:
7.4 = 2.50 + log [pyruvate]/[pyruvic acid]
4.9 = log [pyruvate]/[pyruvic acid]
The antilog of 4.9 = 7.9 x 104 = [pyruvate]/[pyruvic acid]Lactic acid:
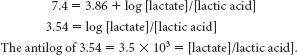
Thus, these organic acids, like most organic acids that we will encounter in our study of biochemistry, are extensively ionized at physiological pH.
Page C26.48
7.8
100
Initial acetate (M)
Initial HCl (M)
pH
0.1
0.0025
6.3
0.1
0.005
6.0
0.1
0.01
5.7
0.1
0.05
4.8
0.01
0.0025
5.2
0.01
0.005
4.8
0.01
0.01
3.4
0.01
0.05
1.4
Acetate ion = 0.128 M; acetic acid = 0.072 M.
First, take a deep breath. You need to determine the concentration of base and acid that will be present in the 0.2 M acetate buffer. Fortunately, you have just completed the previous problem (problem 20), so you know that in the final buffer,
[acetate] = 0.128 M and [acetic acid] = 0.072 M
Because you are making half a liter of buffer, you will need
0.5 l × 0.128 mol l−1 = 0.064 mol of sodium acetate
0.064 mol × 82 g mol−1 = 5.25 g of sodium acetate
You weigh the required amount of sodium acetate.
For the 500-
ml buffer, you will require 0.036 mol of acetic acid. Your acid solution is 1 mol l−1 [1 M], so 1 mol l−1 × 0.036 mol = 0.036 l or 36 ml
To produce the final buffer, dissolve 5.25 g of acetate in some water, add the 36 ml of 1M acetic acid, dilute to 500 ml, and bask in the adoring gaze of your lab mates.
To solve this problem, we resort to the Henderson–
Hasselbalch equation: 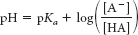
Substituting the given values,
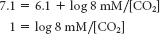
The antilog of 1 is 10; thus,
10 = 8 mM/[CO2]
and
[CO2] = 8 mM/10 = 0.8 mM
Again, we fall back on the Henderson–
Hasselbalch equation: 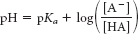
Substituting the values, we arrive at
0.93 = log [HCO3−]/1.10 mM
The antilog of 0.93 = 8.5, so
8.5 = [HCO3−]/1.10 mM
and
[HCO3−] = 9.4, a dangerously low value.
Intravenous administration of sodium bicarbonate is a common treatment.
Middle-
distance running involves aspects of endurance running as well as sprinting. Some studies have shown that drinking a sodium bicarbonate solution prior to the run mitigates a drop in pH and improves performance.