Chapter 20
An oxidizing agent, or oxidant, accepts electrons in oxidation–
reduction reactions. A reducing reagent, or reductant, donates electrons in such reactions. Complete the interactive matching exercise to see answers.
Biochemists use
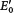 , the value at pH 7, whereas chemists use E0, the value in 1 M H+. The prime denotes that pH 7 is the standard state.
, the value at pH 7, whereas chemists use E0, the value in 1 M H+. The prime denotes that pH 7 is the standard state.ΔG°′ is + 67 kJ mol−1 (+16.1 kcal mol−1) for oxidation by NAD+ and −3.8 kJ mol−1 (−0.92 kcal mol−1) for oxidation by FAD. The oxidation of succinate by NAD+ is not thermodynamically feasible.
The most bacteria-
like mitochondrial genome, that of the protozoan Reclinomonas americana, consist of 97 genes with 62 genes encoding proteins. The protein- coding genes, which comprise only 2% of the protein- coding genes in the bacterium E. coli, include all of the protein- coding genes found in all of the sequenced mitochondrial genomes. Thus, 2% of bacterial genes are found in all examined mitochondria. It seems unlikely that mitochondrial genomes resulting from several endosymbiotic events could have been independently reduced to the same set of genes found in R. americana. The simplest explanation is that the endosymbiotic event took place just once and all existing mitochondria are descendants of that ancestor. Pyruvate accepts electrons and is thus the oxidant. NADH gives up electrons and is the reductant.
The value
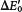 of iron can be altered by changing the environment of the ion.
of iron can be altered by changing the environment of the ion.
The 10 isoprene units render coenzyme Q soluble in the hydrophobic environment of the inner mitochondrial membrane. The two oxygen atoms can reversibly bind two electrons and two protons as the molecule transitions between the quinone form and the quinol form.
c, e, b, a, d
Complete the interactive matching exercise to see answers.
Hydroxyl radical (OH ·), hydrogen peroxide (H2O2), superoxide ion (
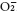 ), and peroxide (O22−). These small molecules react with a host of macromolecules—
), and peroxide (O22−). These small molecules react with a host of macromolecules—including proteins, nucleotides, and membranes— to disrupt cell structure and function. Rotenone: NADH, NADH-
Q oxidoreductase will be reduced. The remainder will be oxidized. Antimycin A: NADH, NADH- Q oxidoreductase and coenzyme Q will be reduced. The remainder will be oxidized. Cyanide: All will be reduced. The respirasome is another example of the use of supramolecular complexes in biochemistry. Having the three complexes that are proton pumps associated with one another will enhance the efficiency of electron flow from complex to complex, which in turn will cause more efficient proton pumping.
Page C21Triose phosphate isomerase converts dihydroxyacetone phosphate (a potential dead end) into glyceraldehyde 3-
phosphate (a mainstream glycolytic intermediate). Vitamins C and E
Exercise induces superoxide dismutase, which converts ROS into hydrogen peroxide and oxygen.
The answer to this question is not fully established. Two possibilities are (1) the suppression of ROS by vitamins prevents the expression of more superoxide dismutase and (2) some ROS may be signal molecules required to stimulate the biochemical benefits of exercise.
Succinate dehydrogenase is a component of Complex II.
In fermentations, organic compounds are both the donors and the acceptors of electrons. In respiration, the electron donor is usually an organic compound, whereas the electron acceptor is an inorganic molecule, such as oxygen.
The ΔG°′ for the reduction of oxygen by FADH2 is −200 kJ mol−1 (−48 kcal mol−1).
This inhibitor (like antimycin A, problem 13) blocks the reduction of cytochrome c1 by QH2, the crossover point.