Chapter 22
Ultimately, all of the carbon atoms of which we are made, not just carbohydrates, enter the biosphere through the process of photosynthesis. Moreover, the oxygen that we require is produced by photosynthesis.
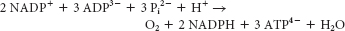
Complete the interactive matching exercise to see answers.
Photosystem II, in conjunction with the oxygen-
generating complex, powers oxygen release. The reaction center of photosystem II absorbs light maximally at 680 nm. Oxygen consumption will be maximal when photosystems I and II are operating cooperatively. Oxygen will be efficiently generated when electrons from photosystem II fill the electron holes in photosystem I, which were generated when the reaction center of photosystem I was illuminated by light of 700 nm.
The light reactions take place on thylakoid membranes. Increasing the membrane surface increases the number of ATP-
and NADH- generating sites. Photoinduced separation of charge results when a high-
energy electron generated by light absorption moves to a neighboring acceptor molecule with a lower excited state. This step is in photosynthesis is fundamental because the now negatively charged acceptor molecule possesses a high- energy electron that can be used to generate a proton gradient or biosynthetic reducing power. These complexes absorb more light than can a reaction center alone. The light-
harvesting complexes funnel light to the reaction centers. NADP+ is the acceptor. H2O is the donor. Light energy powers the electron flow.
The charge gradient, a component of the proton-
motive force in mitochondria, is neutralized by the influx of Mg2+ into the lumen of the thylakoid membranes. Chlorophyll is readily inserted into the hydrophobic interior of the thylakoid membranes.
Chlorophyll contains networks of alternating single and double bonds. Such networks allow electrons to resonate and thus are not held tightly by a particular atom. This situation allows excitation of the electrons by light energy.
Page C23Protons released by the oxidation of water; protons pumped into the lumen by the cytochrome b6f complex; protons removed from the stroma by the reduction of NADP+ and plastoquinone
The electron flow from PS II to PS I is uphill, or endergonic. For this uphill flow, ATP would need to be consumed, defeating the purpose of photosynthesis.
ΔE′;0 = 10.11 V, and ΔG°′ = −21.3 kJ mol−1 (−5.1 kcal mol−1).
All ecosystems require an energy source from outside the system, because the chemical-
energy sources will ultimately be limited. The photosynthetic conversion of sunlight is one example of such a process. Not at all. Spock would point out that chemicals other than water can donate electrons and protons.
DCMU inhibits electron transfer in the link between photosystems II and I. O2 can evolve in the presence of DCMU if an artificial electron acceptor such as ferricyanide can accept electrons from Q.
DCMU will have no effect because it blocks photosystem II, and cyclic photophosphorylation uses photosystem I and the cytochrome b6f complex.
Exposure to light allowed the generation of a proton gradient, but the absence of ADP and Pi prevented the synthesis of ATP. When the chloroplasts were placed in a dark environment with ADP and Pi, ATP synthesis could take place until the gradient was depleted.
Oxygen generation would stop. The proton gradient generated by photosystem II would not be dissipated. Soon the gradient would become so great that the energy of electron flow in photosystem II would be incapable of pumping more protons. Oxygen generation (as well as photosystem I) would halt.
An uncoupler, such as dinitrophenol
The cristae
In eukaryotes, both processes take place in specialized organelles. Both depend on high-
energy electrons to generate ATP. In oxidative phosphorylation, the high- energy electrons originate in fuels and are extracted as reducing power in the form of NADH. In photosynthesis, the high- energy electrons are generated by light and are captured as reducing power in the form of NADPH. Both processes use redox reactions to generate a proton gradient, and the enzymes that convert the proton gradient into ATP are similar in both processes. In both systems, electron transport takes place in membranes inside organelles. Both enzymes transfer electrons from FADH2 to a nicotinamide nucleotide: NADP+ in the reductase and NAD+ in the dehydrogenase complex. Such electron flow is uncommon. Usually, electrons flow from reduced nicotinamide nucleotide to FAD.
Both photosynthesis and cellular respiration are powered by high-
energy electrons flowing toward a more stable state. In cellular respiration, the high- energy electrons are derived from the oxidation of carbon fuels as NADH and FADH2. They release their energy as they reduce oxygen. In photosynthesis, high- energy electrons are generated by absorbing light energy, and they find stability in photosystem 1 and ferredoxin. Thioredoxin
The control enzyme is unaffected, but the mitochondrial enzyme with part of the chloroplast γ subunit increases activity as the concentration of DTT increases.
The increase was even larger when thioredoxin was present. Thioredoxin is the natural reductant for the chloroplast enzyme, and so it presumably operates more efficiently than would DTT, which probably functions to keep the thioredoxin reduced.
They seem to have done so.
The enzyme is susceptible to control by the redox state. In plant cells, reduced thioredoxin is generated by photosystem I. Thus, the enzyme is active when photosynthesis is taking place.
Cysteine
Group-
specific modification or site- specific mutagenesis
The absorption of light by photosystem I results in a ΔE′0 of −1.0 V. Recall that ΔG′0 = −nFΔE′0, where F = 96.48 kJ mol−1 V−1. Under standard conditions, the energy change for the electrons is 96.5 kJ. Thus, the efficiency is 96.5/172 = 56%.
120 kJ einstein−1 (28.7 kcal einstein−1)
1.24 V
One 1000-
nm photon has the free- energy content of 2.4 molecules of ATP. A minimum of 0.42 photon is needed to drive the synthesis of a molecule of ATP.
The electrons flow through photosystem II directly to ferricyanide. No other steps are required.
NADPH must be factored in because it is an energy-
rich molecule. Recall from Chapter 21, that NADH is worth 2.5 ATP if oxidized by the electron- transport chain. Thus, 12 NADPH = 30 ATP. Eighteen ATP are used directly, so the equivalent of 48 molecules of ATP are required for the synthesis of glucose.