Chapter 27
In stage 1, triacylglycerols are degraded to fatty acids and glycerol, which are released from the adipose tissue and transported to the energy-
requiring tissues. In stage 2, the fatty acids are activated and transported into mitochondria for degradation. In stage 3, the fatty acids are broken down in a step- by- step fashion into acetyl CoA, which is then processed in the citric acid cycle. Glucagon and epinephrine trigger 7TM receptors in adipose tissue that activate adenylate cyclase. The increased level of cyclic AMP then stimulates protein kinase A, which phosphorylates two key proteins: perilipin, a fat-
droplet- associated protein, and hormone- sensitive lipase. The phosphorylation of perilipin restructures the fat droplet so that the triacylglycerols are more readily mobilized, and it triggers the release of a coactivator for the adipose triglyceride lipase (ATGL). Activated ATGL then initiates the mobilization of triacylglycerols by releasing a fatty acid from triacylglycerol, forming diacylglycerol. Diacylglycerol is converted into a free fatty acid and monoacylglycerol by the hormone- sensitive lipase. Monoacylglycerol lipase completes the mobilization of fatty acids with the production of a free fatty acid and glycerol. The ready reversibility is due to the high-
energy nature of the thioester in the acyl CoA. 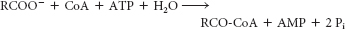
To return the AMP to a form that can be phosphorylated by oxidative phosphorylation or substrate-
level phosphorylation, another molecule of ATP must be expended in the reaction: ATP + AMP ⇌ 2 ADP
Oxidation by flavin adenine dinucleotide (FAD), hydration, oxidation by nicotinamide adenine dinucleotide (NAD+), and thiolysis by coenzyme A.
Complete the interactive matching exercise to see answers.
b, c, a, g, h, d, e, f
Fatty acids cannot be transported into mitochondria for oxidation. The muscles could not use fats as a fuel. Muscles could use glucose derived from glycogen. However, when glycogen stores are depleted, as after a fast, the effect of the deficiency is especially apparent.
The next-
to- last degradation product, acetoacetyl CoA, yields two molecules of acetyl CoA with the thiolysis by only one molecule of CoA. Palmitic acid yields 106 molecules of ATP. Palmitoleic acid has a double bond between carbons C-
9 and C- 10. When palmitoleic acid is processed in β oxidation, one of the oxidation steps (to introduce a double bond before the addition of water) will not take place, because a double bond already exists. Thus, FADH2 will not be generated, and palmitoleic acid will yield 1.5 fewer molecules of ATP than palmitic acid, for a total of 104.5 molecules of ATP. Activation fee to form the acyl CoA
−2
ATP
Seven rounds of yield:
7 acetyl CoA at 10 ATP/acetyl CoA
+70
ATP
7 NADH at 2.5 ATP/NADH
+17.5
ATP
7 FADH2 at 1.5 ATP/FADH2
+10.5
ATP
Propionyl CoA, which requires an ATP to be converted into succinyl CoA
−1
ATP
Succinyl CoA → succinate
+1
ATP
Succinate → fumarate + FADH2 FADH2 at 1.5 ATP/FADH2
+1.5
ATP
Fumarate → malate
Malate → oxaloacetate + NADH NADH at 2.5 ATP/NADH
+2.5
ATP
Total
100
ATP
You might hate yourself in the morning, but at least you won’t have to worry about energy. To form stearoyl CoA requires the equivalent of 2 molecules of ATP.
Page C28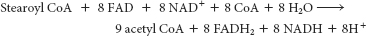
9 acetyl CoA at 10 ATP/acetyl CoA
+90
ATP
8 NADH at 2.5 ATP/NADH
+20
ATP
8 FADH2 at 1.5 ATP/FADH2
+12
ATP
Activation fee
−2.0
Total
122
ATP
After a night’s sleep, glycogen stores will be low, but fats will be plentiful. Muscles will burn fat as a fuel. Why the caffeine? Lipid mobilization is stimulated by glucagon and epinephrine, and both of these hormones can work through the cAMP cascade. cAMP stimulates protein kinase A, which stimulates the breakdown of triacylglycerols in the adipose tissue. If cAMP hydrolysis to AMP is inhibited, protein kinase A will be maximally stimulated, fat will be maximally mobilized, and you will look as buff and lean as a svelte waterfowl.
Keep in mind that, in the citric acid cycle, 1 molecule of FADH2 yields 1.5 molecules of ATP, 1 molecule of NADH yields 2.5 molecules of ATP, and 1 molecule of acetyl CoA yields 10 molecules of ATP. Two molecules of ATP are produced when glucose is degraded to 2 molecules of pyruvate. Two molecules of NADH also are produced, but the electrons are transferred to FADH2 to enter mitochondria. Each molecule of FADH2 can generate 1.5 molecules of ATP. Each molecule of pyruvate will produce 1 molecule of NADH. Each molecule of acetyl CoA generates 3 molecules of NADH, 1 molecule of FADH2, and 1 molecule of ATP. So, we have a total of 10 molecules of ATP per molecule of acetyl CoA, or 20 for the 2 molecules of acetyl CoA. The total for glucose is 30 ATP. Now, what about hexanoic acid? Caproic acid is activated to caproic CoA at the expense of 2 ATP, and so we are 2 ATP in the hole. The first cycle of β oxidation generates 1 FADH2, 1 NADH, and 1 acetyl CoA. After the acetyl CoA has been run through the citric acid cycle, this step will have generated a total of 14 ATP. The second cycle of β oxidation generates 1 FADH2 and 1 NADH but 2 acetyl CoA. After the acetyl CoA has been run through the citric acid cycle, this step will have generated a total of 24 ATP. The total is 36 ATP. Thus, the foul-
smelling caproic acid has a net yield of 36 ATP. So on a per carbon basis, this fat yields 20% more ATP than does glucose, a manifestation of the fact that fats are more reduced than carbohydrates. 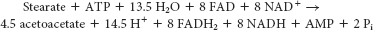
Palmitate is activated and then processed by β oxidation according to the following reactions:
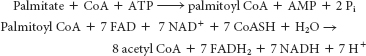
The 8 molecules of acetyl CoA combine to form 4 molecules of acetoacetate for release into the blood, and so they do not contribute to the energy yield in the liver. However, the FADH2 and NADH generated in the preparation of acetyl CoA can be processed by oxidative phosphorylation to yield ATP.
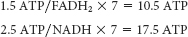
The equivalent of 2 ATP were used to form palmitoyl CoA. Thus, 26 ATP were generated for use by the liver.
NADH produced with the oxidation to acetoacetate = 2.5 ATP Acetoacetate is converted into acetoacetyl CoA.
Two molecules of acetyl CoA result from the hydrolysis of acetoacetyl CoA, each worth 10 ATP when processed by the citric acid cycle. Total ATP yield is 22.5.
Because a molecule of succinyl CoA is used to form acetoacetyl CoA. Succinyl CoA could be used to generate one molecule of ATP, and so someone could argue that the yield is 21.5.
For fats to be combusted, not only must they be converted into acetyl CoA, but the acetyl CoA must be processed by the citric acid cycle. In order for acetyl CoA to enter the citric acid cycle, there must be a supply of oxaloacetate. Oxaloacetate can be formed by the metabolism of glucose to pyruvate and the subsequent carboxylation of pyruvate to form oxaloacetate.
The absence of ketone bodies is due to the fact that the liver, the source of ketone bodies in the blood, cannot oxidize fatty acids to produce acetyl CoA. Moreover, because of the impaired fatty acid oxidation, the liver becomes more dependent on glucose as an energy source. This dependency results in a decrease in gluconeogenesis and a drop in blood-
glucose levels, which is exacerbated by the lack of fatty acid oxidation in muscle and a subsequent increase in glucose uptake from the blood. Liver cells lack the specific CoA transferase that converts acetoacetate into acetoacetyl CoA, which is subsequently cleaved into two molecules of acetyl CoA. The lack of the enzyme allows liver to produce ketone bodies but not to use them.
In the absence of insulin, lipid mobilization will take place to an extent that it overwhelms the ability of the liver to convert the lipids into ketone bodies.
Two carbon atoms enter the cycle as an acetyl group, but two carbons leave the cycle as CO2 before oxaloacetate is generated. Consequently, no net synthesis of oxaloacetate is possible. In contrast, plants have two additional enzymes enabling them to convert the carbon atoms of acetyl CoA into oxaloacetate in the glyoxylate cycle.
The carbon skeletons of the released amino acids are used to synthesize glucose for use by the brain and red blood cells.
Muscle shifts from glucose to fatty acids for fuel. This switch lessens the need to degrade protein for glucose formation. The degradation of fatty acids by muscle halts the conversion of pyruvate into acetyl CoA, because acetyl CoA derived from fatty acids inhibits pyruvate dehydrogenase, the enzyme that converts pyruvate into acetyl CoA.

Glycerol kinase and glycerol phosphate dehydrogenase
The citric acid cycle. The reactions that take succinate to oxaloacetate, or the reverse, are similar to those of fatty acid metabolism.
Fats burn in the flame of carbohydrates. Without carbohydrates, there would be no anapleurotic reactions to replenish the components of the citric acid cycle. With a diet of fats only, the acetyl CoA from fatty acid degradation would build up.
Acetone from ketone bodies
Yes. Odd-
chain fatty acids would lead to the production of propionyl CoA, which can be converted into succinyl CoA, a citric acid cycle component. It would serve to replenish the citric acid cycle and mitigate the halitosis.
The Vmax is decreased, and the KM is increased. Vmax (wild type) = 13 nmol minute−1 mg−1; KM (wild type) = 45 µM; Vmax (mutant) = 8.3 nmol minute−1 mg−1; KM (mutant) = 74 µM.
Both the Vmax and the KM are decreased. Vmax (wild type) = 41 nmol minute−1 mg−1; KM (wild type) = 104 µM; Vmax (mutant) = 23 nmol minute−1 mg−1; KM (mutant) = 69 µM.
The wild type is significantly more sensitive to malonyl CoA.
Page C29With respect to carnitine, the mutant displays approximately 65% of the activity of the wild type; with respect to palmitoyl CoA, approximately 50% activity. On the other hand, 10 µM of malonyl CoA inhibits approximately 80% of the wild type but has essentially no effect on the mutant enzyme.
The glutamate appears to play a more prominent role in regulation by malonyl CoA than in catalysis.
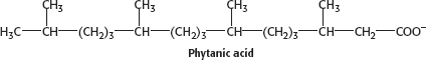
The problem with phytanic acid is that, as it undergoes β oxidation, we encounter the dreaded pentavalent carbon atom. Because the pentavalent carbon atom doesn’t exist, β oxidation cannot take place and phytanic acid accumulates.
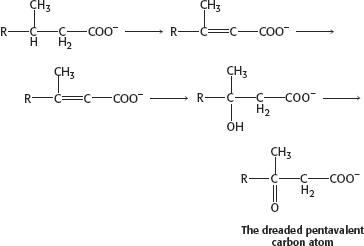
How do we solve the problem? Well, the removal of methyl groups, though theoretically possible, would be time consuming and, well, lacking in elegance. What would we do with the methyl groups? We solve this problem—
well, actually our livers solve the problem— by inventing α oxidation. 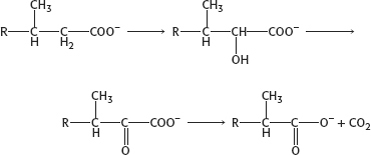
One round of α oxidation rather than β oxidation converts phytanic acid into a β-oxidation substrate.
Radioactive lipids are combusted to acetyl CoA, which is metabolized by the citric acid cycle. However, the two carbon atoms that enter the cycle as acetyl CoA are not the two carbon atoms that leave the cycle as CO2. Consequently, some 14C will appear in oxaloacetate, which can be converted into glucose and then into glycogen.
Eventually all of the CoA would be in the form of acetyl CoA, and no energy could be produced. Moreover, the high concentrations of acetyl CoA would eventually inhibit fatty acid oxidation (not to mention glucose oxidation). Getting rid of the acetyl CoA (generating ketone bodies) allows for the generation of high-
energy electrons by fatty acid oxidation and for the provision of fuel for other tissues. The first oxidation removes two tritium atoms. The hydration adds nonradioactive H and OH. The second oxidation removes another tritium atom from the β-carbon atom. Thiolysis removes an acetyl CoA with only one tritium; so the tritium-
to- carbon ratio is 1/2. This ratio will be the same for two of the acetates. The last one, however, does not undergo oxidation, and so all tritium remains. The ratio for this acetate is 3/2. The ratio for the entire molecule is then 5/6.