Chapter 31
Nitrogen fixation is the conversion of atmospheric N2 into NH4+ Diazotrophic (nitrogen-
fixing) microorganisms are responsible for this reaction. 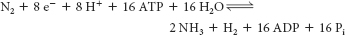
The fixation of nitrogen is an exergonic reaction. The role of ATP is to reduce the activation energy of the barriers in the reaction pathway so as to render the reaction kinetically feasible.
Complete the interactive matching exercise to see answers.
Page C33The reductase provides electrons with high reducing power, whereas the nitrogenase, which requires ATP hydrolysis, uses the electrons to reduce N2 to NH3.
False. Nitrogen is thermodynamically favored. ATP expenditure by the nitrogenase is required to make the reaction kinetically possible.
The bacteria provide the plant with ammonia by reducing atmospheric nitrogen. This reduction is energetically expensive, and the bacteria use ATP from the plant.
Human beings do not have the biochemical pathways to synthesize essential amino acids from simpler precursors. Consequently, they must be obtained from the diet.
Oxaloacetate, pyruvate, ribose-
5- phosphate, phosphoenolpyruvate, erythrose- 4- phosphate, α-ketoglutarate, and 3- phosphoglycerate Pyridoxal phosphate (PLP)
A reversible transamination reaction will transfer the labeled amino group from aspartate to α-ketoglutarate to form glutamate and oxaloacetate. Glutamate is an amino-
group donor for the synthesis of many amino acids from their corresponding ketoacids. Tetrahydrofolate is a carrier of a variety of one-
carbon units. Both carry one-
carbon units. S-Adenosylmethionine is a more useful methyl donor than tetrahydrofolate because it has a greater transfer potential. γ-Glutamyl phosphate is a likely reaction intermediate.
Alanine from pyruvate; aspartate from oxaloacetate; glutamate from α-ketoglutarate
Y could inhibit the C → D step, Z could inhibit the C → F step, and C could inhibit A →B. This scheme is an example of sequential feedback inhibition. Alternatively, Y could inhibit the C → D step, Z could inhibit the C → F step, and the A → B step would be inhibited only in the presence of both Y and Z. This scheme is called concerted feedback inhibition.
The rate of the A → B step in the presence of high levels of Y and Z would be 24 s−1 (0.6 × 0.4 × 100 s−1).
Tetrahydrofolate, S-adenosylmethionine, and biotin
Methylmalonyl CoA mutase, an enzyme required for the degradation of odd-
chain fatty acids Methionine is a component of S-adenosylmethionine, which is a methyl donor for the synthesis of phosphatidylcholine from phosphatidylethanolamine.
Asparagine is much more abundant in the dark. More glutamine is present in the light. These amino acids show the most dramatic effects. Glycine also is more abundant in the light.
Glutamine is a more metabolically reactive amino acid, used in the synthesis of many other compounds. Consequently, when energy is available as light, glutamine will be preferentially synthesized. Asparagine, which carries more nitrogen per carbon atom and is thus a more efficient means of storing nitrogen when energy is short, is synthesized in the dark. Glycine is more prevalent in the light because of photorespiration.
White asparagus has an especially high concentration of asparagine, which accounts for its intense taste. All asparagus has a large amount of asparagine. In fact, as suggested by its name, asparagine was first isolated from asparagus.
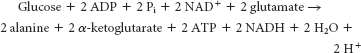
Synthesis from oxaloacetate and α-ketoglutarate would deplete the citric acid cycle, which would decrease ATP production. Anapleurotic reactions would be required to replenish the citric acid cycle.
The value of KM of glutamate dehydrogenase for NH4+ is high (≫1 mM), and so this enzyme is not saturated when NH4+ is limiting. In contrast, glutamine synthetase has very low KM for NH4+. Thus, ATP hydrolysis is required to capture ammonia when it is scarce.