Chapter 32
In de novo synthesis, the nucleotides are synthesized from simpler precursor compounds, in essence from scratch. In salvage pathways, preformed bases are recovered and attached to activated riboses.
(a and c) PRPP; (b) carbamoyl phosphate
A nucleoside is a base attached to ribose. A nucleotide is a nucleoside with the ribose bearing one or more phosphates.
Complete the interactive matching exercise to see answers.
dUMP + serine + NADPH + H+ → TMP + NADP+ + glycine
Sulfanilamide inhibits the synthesis of folate by acting as an analog of p-aminobenzoate, one of the precursors of folate. This results in a deficiency of N10-formyltetrahydrofolate.
PRPP is the activated intermediate in the synthesis of phosphoribosylamine in the de novo pathway of purine formation; in the synthesis of purine nucleotides from free bases by the salvage pathway; and in the synthesis of orotidylate in the formation of pyrimidines.
The reciprocal substrate relation refers to the fact that AMP synthesis requires GTP, whereas GMP synthesis requires ATP. This relation tends to balance the synthesis of ATP and GTP.
The synthesis of carbamoyl phosphate requires 2 ATP
2 ATP
The formation of PRPP from ribose 5-
hosphate yields 1 AMP* 2 ATP
The conversion of UMP to UTP requires 2 ATP
2 ATP
The conversion of UTP to CTP requires 1 ATP
1 ATP
Total
7 ATP
*Remember that 1 AMP is the equivalent of 2 ATP because an ATP must be expended to generate ADP the substrate for ATP synthesis.
The label will be on C-
6 for cytosine and on C- 5 for guanine, to correspond to 13C labels on the α carbons of aspartate and glycine, respectively. N-
3 and N- 9 in the purine ring (a) Carboxyaminoimidazole ribonucleotide; (b) glycinamide ribonucleotide; (c) phosphoribosylamine; (d) formylglycinamide ribonucleotide
Allopurinol, an analog of hypoxanthine, is a suicide inhibitor of xanthine oxidase.
Leukemia patients have a high level of urate because of the breakdown of nucleic acids due to cell death. Allopurinol prevents the formation of kidney stones and blocks other deleterious consequences of hyperuricemia by preventing the formation of urate.
Because folate is required for nucleotide synthesis, cells that are dividing rapidly would be most readily affected. They would include cells of the intestine, which are constantly replaced, and precursors to blood cells. The lack of intestinal cells and blood cells would account for the symptoms often observed.
Inosine or hypoxanthine could be administered.
N-
1 in both cases, and the amine group linked to C- 6 in ATP. By their nature, cancer cells divide rapidly and thus require frequent DNA synthesis. Inhibitors of TMP synthesis will impair DNA synthesis and cancer growth.
Page C34(a) cAMP; (b) ATP; (c) UDP-
glucose; (d) acetyl CoA; (e) NAD+, FAD; (f) fluorouracil; (g) CTP inhibits ATCase. (a) Some ATP can be salvaged from the ADP that is being generated. (b) There are equal numbers of high-
phosphoryl- transfer- potential groups on each side of the equation. (c) Because the adenylate kinase reaction is at equilibrium, the removal of AMP would lead to the formation of more ATP. (d) Essentially, the cycle serves as an anapleurotic reaction for the generation of the citric acid cycle intermediate fumarate. Carbamoyl phosphate I uses ammonia to synthesize carbamoyl phosphate for a reaction with ornithine, the first step of the urea cycle. Carbamoyl phosphate II uses glutamine to synthesize carbamoyl phosphate for use in the first step of pyrimidine biosynthesis.
Allopurinol is an inhibitor of xanthine oxidase, which is on the pathway for urate synthesis. In your pet duck, this pathway is the means by which excess nitrogen is excreted. If xanthine oxidase were inhibited in your duck, nitrogen could not be excreted, with severe consequences such as a dead duck.
PRPP and formylglycinamide ribonucleotide
Cell A cannot grow in a HAT medium, because it cannot synthesize TMP either from thymidine or from dUMP. Cell B cannot grow in this medium, because it cannot synthesize purines by either the de novo pathway or the salvage pathway. Cell C can grow in a HAT medium because it contains active thymidine kinase from cell B (enabling it to phosphorylate thymidine to TMP) and hypoxanthine guanine phosphoribosyltransferase from cell A (enabling it to synthesize purines from hypoxanthine by the salvage pathway).
Transform cell A with a plasmid containing foreign genes of interest and a functional thymidine kinase gene. The only cells that will grow in a HAT medium are those that have acquired a thymidylate kinase gene; nearly all of these transformed cells will also contain the other genes on the plasmid.
The cytoplasmic level of ATP in the liver falls and that of AMP rises above normal in all three conditions. The excess AMP is degraded to urate.
Succinate → malate → oxaloacetate by the citric acid cycle. Oxaloacetate → aspartate by transamination, followed by pyrimidine synthesis. Carbons 4, 5, and 6 are labeled.
Glucose will most likely be converted into two molecules of pyruvate, one of which will be labeled in the 2 position:
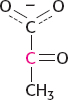
Now consider two common fates of pyruvate—
conversion into acetyl CoA and subsequent processing by the citric acid cycle or carboxylation by pyruvate carboxylase to form oxaloacetate. The formation of citrate by condensing the labeled acetyl CoA (derived from labeled pyruvate) with oxaloacetate will yield labeled citrate:
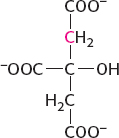
The labeled carbon atom will be retained through one round of the citric acid cycle, but, on the formation of the symmetric succinate, the label will appear in two different positions. Thus, when succinate is metabolized to oxaloacetate, which may be aminated to form aspartate, two carbons will be labeled:
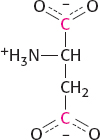
When this aspartate is used to form uracil, the labeled COO− attached to the α-carbon is lost and the other COO− becomes incorporated into uracil as carbon 4.
Suppose instead that pyruvate labeled in the 2 position is carboxylated to form oxaloacetate and processed to form aspartate. In this case, the α-carbon of aspartate bears the label.
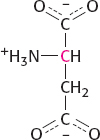
When this aspartate is used to synthesize uracil, carbon 6 bears the label.