Chapter 41
A cDNA library is set of DNA segments complementary to mRNA sequences from a cell type. The mRNA is isolated and converted into double-
stranded DNA with the use of reverse transcriptase and DNA polymerase. The double- stranded DNA is ligated to linkers, which are then inserted into some sort of vector. A cDNA library is a DNA representation of the mRNA expressed in a particular tissue under a specific set of physiological conditions. cDNA libraries vary from cell type to cell type from the same organism. A genomic library is a collection of DNA fragments, inserted into vector molecules, that represent the entire genome of an organism. Genomic libraries prepared from any diploid tissue in an organism are identical.
Taq polymerase is the DNA polymerase from the thermophilic bacterium that lives in hot springs. Consequently, it is heat stable and can withstand the high temperatures required for PCR without denaturing.
Complete the interactive matching exercise to see answers.
The codon(s) for each amino acid can be used to determine the number of possible nucleotide sequences that encode each peptide sequence (Table 4.5):

The set of DNA sequences encoding the peptide Gly-
Trp- Asp- Met- His- Lys would be most ideal for probe design because it encompasses only 32 total oligonucleotides. Ovalbumin cDNA should be used. E. coli lacks the machinery to splice the primary transcript arising from genomic DNA.
The presence of the AluI sequence would, on average, be (1/4)4, or 1/256, because the likelihood of any base being at any position is one-
fourth and there are four positions. By the same reasoning, the presence of the NotI sequence would be (1/4)8, or 1/65,536. Thus, the average product of digestion by AluI would be 250 base pairs (0.25 kbp) in length, whereas that for NotI would be 66,000 base pairs (66 kbp) in length. No, because most human genes are much longer than 4 kb. A fragment would contain only a small part of a complete gene.
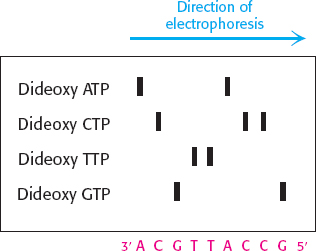
5′-GGCATAC-
3′ The Sanger dideoxy method of sequencing would give the gel pattern shown here.
Southern blotting of an MstII digest would distinguish between the normal and the mutant genes. The loss of a restriction site would lead to the replacement of two fragments on the Southern blot by a single longer fragment. Such a finding would not prove that GTG replaced GAG; other sequence changes at the restriction site could yield the same result.
Because PCR can amplify as little as one molecule of DNA, statements claiming the isolation of ancient DNA need to be greeted with some skepticism. The DNA would need to be sequenced. Is it similar to human, bacterial, or fungal DNA? If so, contamination is the likely source of the amplified DNA. Is it similar to the DNA of birds or crocodiles? This sequence similarity would strengthen the case that it is dinosaur DNA because these species are evolutionarily close to dinosaurs.
At high temperatures of hybridization, only very close matches between primer and target would be stable because all (or most) of the bases would need to find partners to stabilize the primer–
target helix. As the temperature is lowered, more mismatches would be tolerated; so the amplification is likely to yield genes with less sequence similarity. In regard to the yeast gene, synthesize primers corresponding to the ends of the gene, and then use these primers and human DNA as the target. If nothing is amplified at 54°C, the human gene differs from the yeast gene, but a counterpart may still be present. Repeat the experiment at a lower temperature of hybridization. The encoded protein contains four repeats of a specific sequence.
Within a single species, individual dogs show enormous variation in body size and substantial diversity in other physical characteristics. Therefore, genomic analyses of individual dogs would provide valuable clues concerning the genes responsible for the diversity within the species.
If the melting temperatures of the primers are too different, the extent of hybridization with the target DNA will differ during the annealing phase, which would result in differential replication of the strands.
Chongqing: residue 2, L → R, CTG → CGG
Karachi: residue 5, A → P, GCC → CCC
Swan River: residue 6, D → G, GAC → GGC
This particular person is heterozygous for this particular mutation: one allele is wild type, whereas the other carries a point mutation at this position. Both alleles are PCR amplified in this experiment, yielding the “dual peak” appearance on the sequencing chromatogram.
A mutation in person B has altered one of the alleles for gene X, leaving the other intact. The fact that the mutated allele is smaller suggests that it has undergone a deletion. The one functioning allele is transcribed and translated and apparently produces enough protein to render the person asymptomatic.
Person C has only the smaller version of the gene. This gene is neither transcribed (negative northern blot) nor translated (negative western blot).
Person D has a normal-
size allele of the gene but no corresponding RNA or protein. There may be a mutation in the promoter region of the gene that prevents transcription. Person E has a normal-
size allele of the gene that is transcribed, but no protein is made, which suggests that a mutation prevents translation. There are a number of possible explanations, including a mutation that introduced a premature Stop codon in the mRNA. Person F has a normal amount of protein but still displays the metabolic problem. This finding suggests that the mutation affects the activity of the protein—
for instance, a mutation that compromises the active site of enzyme Y. Page C42A simple strategy for generating many mutants is to synthesize a degenerate set of the 30-
bp sequence by using a mixture of activated nucleosides in particular rounds of oligonucleotide synthesis. Suppose that the 30- bp coding region begins with GTT, which encodes valine. If a mixture of all four nucleotides is used in the first and second rounds of synthesis, the resulting oligonucleotides will begin with the sequence XYT (where X and Y denote A, C, G, or T). These 16 different versions of the cassette will encode proteins containing either Phe, Leu, Ile, Val, Ser, Pro, Thr, Ala, Tyr, His, Asn, Asp, Cys, Arg, or Gly at the first position. Likewise, degenerate cassettes can be made in which two or more codons are simultaneously varied. Digest genomic DNA with a restriction enzyme, and select the fragment that contains the known sequence. Circularize this fragment. Then carry out PCR with the use of a pair of primers that serve as templates for the synthesis of DNA away from the known sequence.
Use chemical synthesis or the polymerase chain reaction to prepare hybridization probes that are complementary to both ends of the known (previously isolated) DNA fragment. Challenge clones representing the library of DNA fragments with both of the hybridization probes. Select clones that hybridize to one of the probes but not the other; such clones are likely to represent DNA fragments that contain one end of the known fragment along with the adjacent region of the particular chromosome.