10.2 Monosaccharides Are Linked to Form Complex Carbohydrates
Because sugars contain many hydroxyl groups, glycosidic bonds can join one monosaccharide to another. Oligosaccharides are built by the linkage of two or more monosaccharides by O-glycosidic bonds (Figure 10.8). In the disaccharide maltose, for example, two d-glucose residues are joined by a glycosidic linkage between the α-anomeric form of C-
Specific Enzymes Are Responsible for Oligosaccharide Assembly
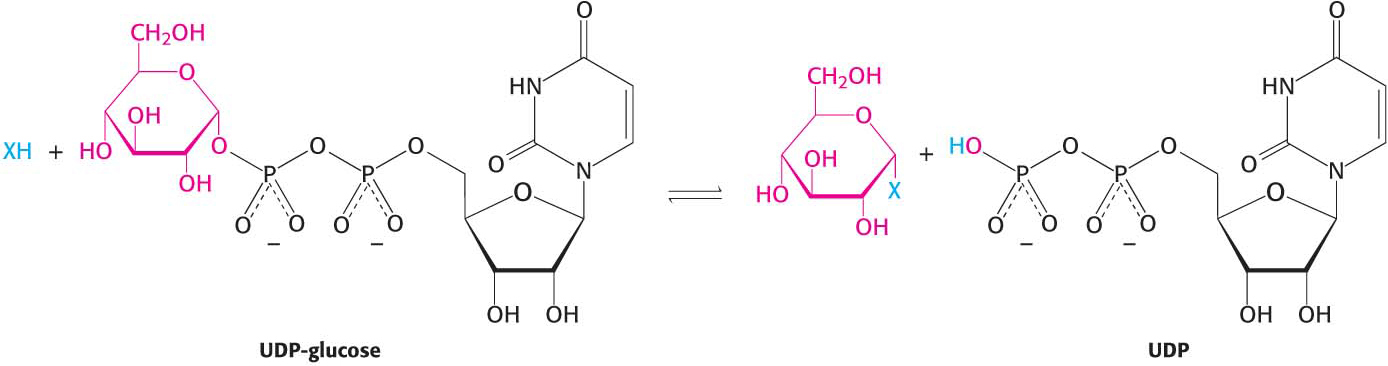
Oligosaccharides are synthesized through the action of specific enzymes, glycosyltransferases, which catalyze the formation of glycosidic bonds. Given the diversity of known glycosidic linkages, many different enzymes are required. Indeed, glycosyltransferases account for 1% to 2% of gene products in all organisms examined.
The general form of the reaction catalyzed by a glycosyltransferase is shown in Figure 10.9. The sugar to be added comes in the form of an activated (energy-
Sucrose, Lactose, and Maltose Are the Common Disaccharides
A disaccharide consists of two sugars joined by an O-glycosidic bond. Three abundant disaccharides that we encounter frequently are sucrose, lactose, and maltose (Figure 10.10). Sucrose (common table sugar) is obtained commercially from sugar cane or sugar beets. The anomeric carbon atoms of a glucose unit and a fructose unit are joined in this disaccharide; the configuration of this glycosidic linkage is a for glucose and β for fructose. Sucrose can be cleaved into its component monosaccharides by the enzyme sucrase.
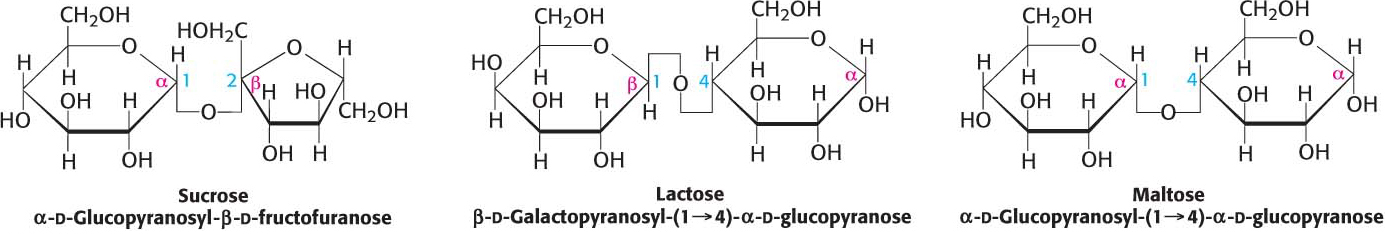
Lactose, the disaccharide of milk, consists of galactose joined to glucose by a β-1,4-
Glycogen and Starch Are Storage Forms of Glucose
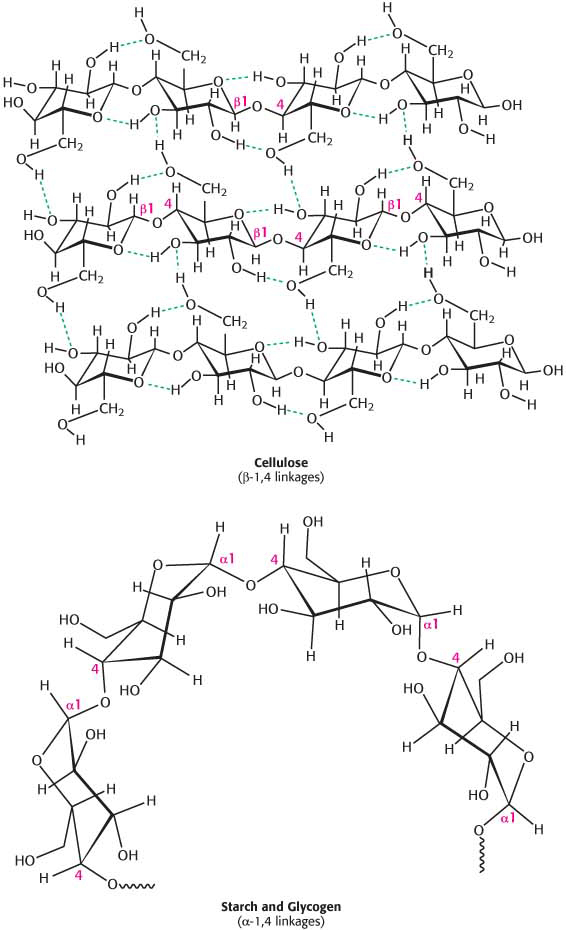
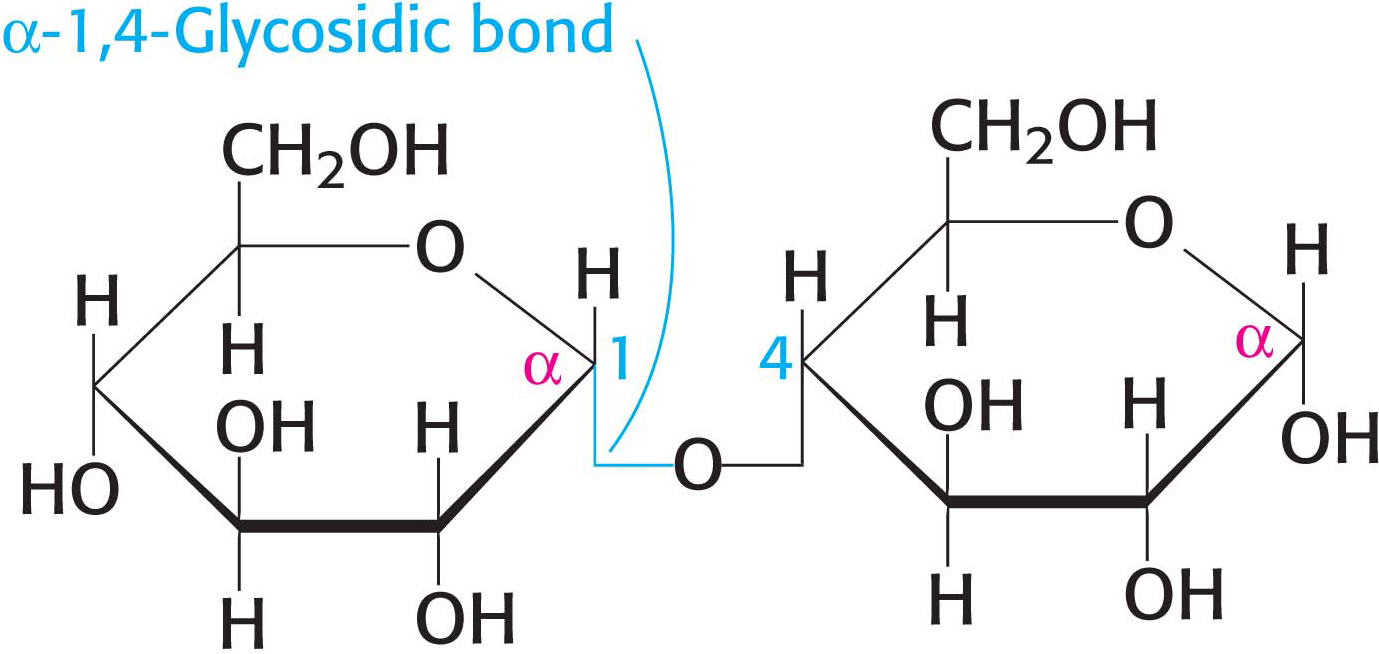
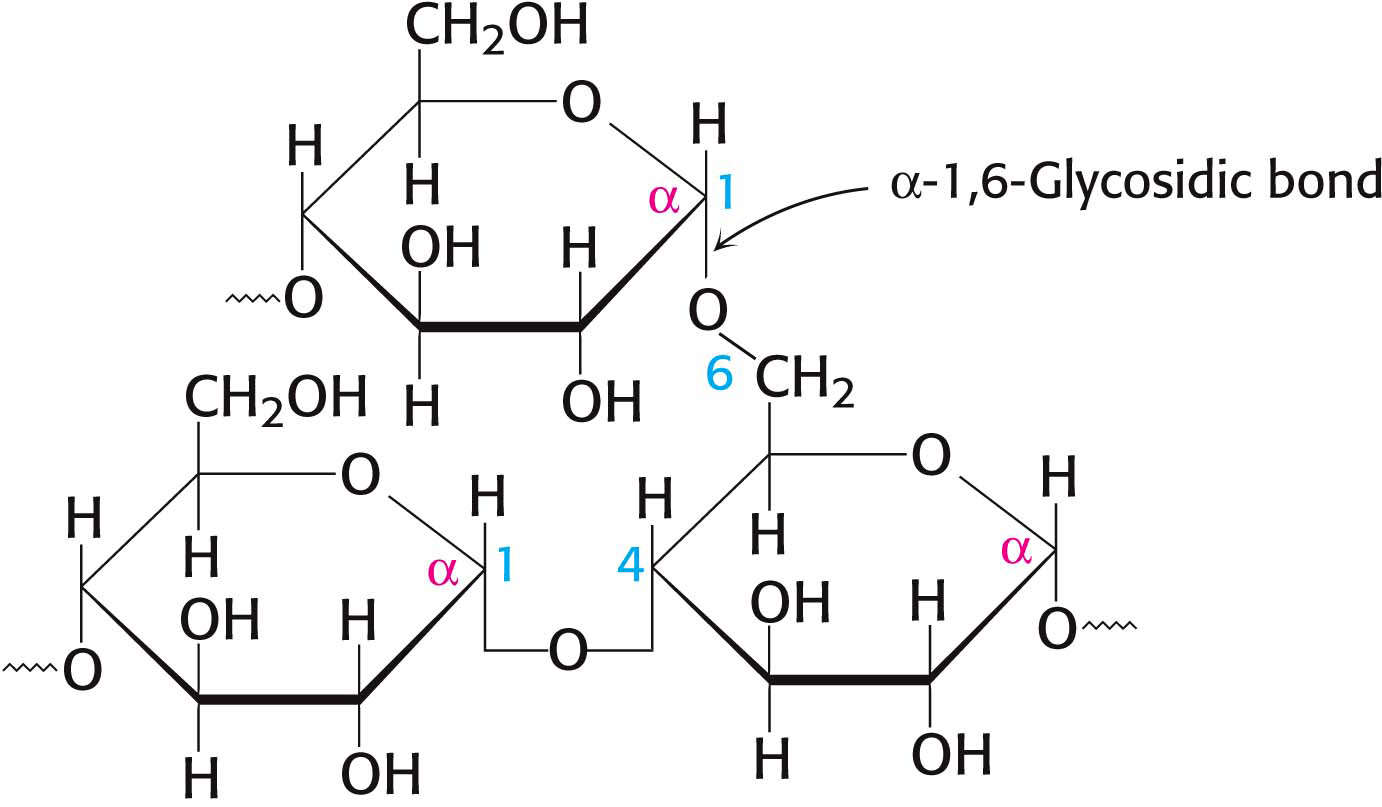
Large polymeric oligosaccharides, formed by the linkage of multiple monosaccharides, are called polysaccharides and play vital roles in energy storage and in maintaining the structural integrity of an organism. The variety of different monosaccharides can be put together in any number of arrangements, creating a huge array of possible polysaccharides. If all of the monosaccharide units in a polysaccharide are the same, the polymer is called a homopolymer. The most common homopolymer in animal cells is glycogen, the storage form of glucose. Glycogen is present in most of our tissues but is most common in muscle and liver. As will be considered in detail in Chapters 24 and 25, glycogen is a large, branched polymer of glucose residues. Most of the glucose units in glycogen are linked by α-1,4-
The nutritional reservoir in plants is the homopolymer starch, of which there are two forms. Amylose, the unbranched type of starch, consists of glucose residues in α-1,4 linkage. Amylopectin, the branched form, has about 1 α-1,6 linkage per 30 α-1,4 linkages, in similar fashion to glycogen except for its lower degree of branching. More than half the carbohydrate ingested by human beings is starch, found in wheat, potatoes, and rice, to name just a few sources (Figure 10.13). Amylopectin, amylose, and glycogen are rapidly hydrolyzed by α-amylase, an enzyme secreted by the salivary glands and the pancreas.
QUICK QUIZ 1
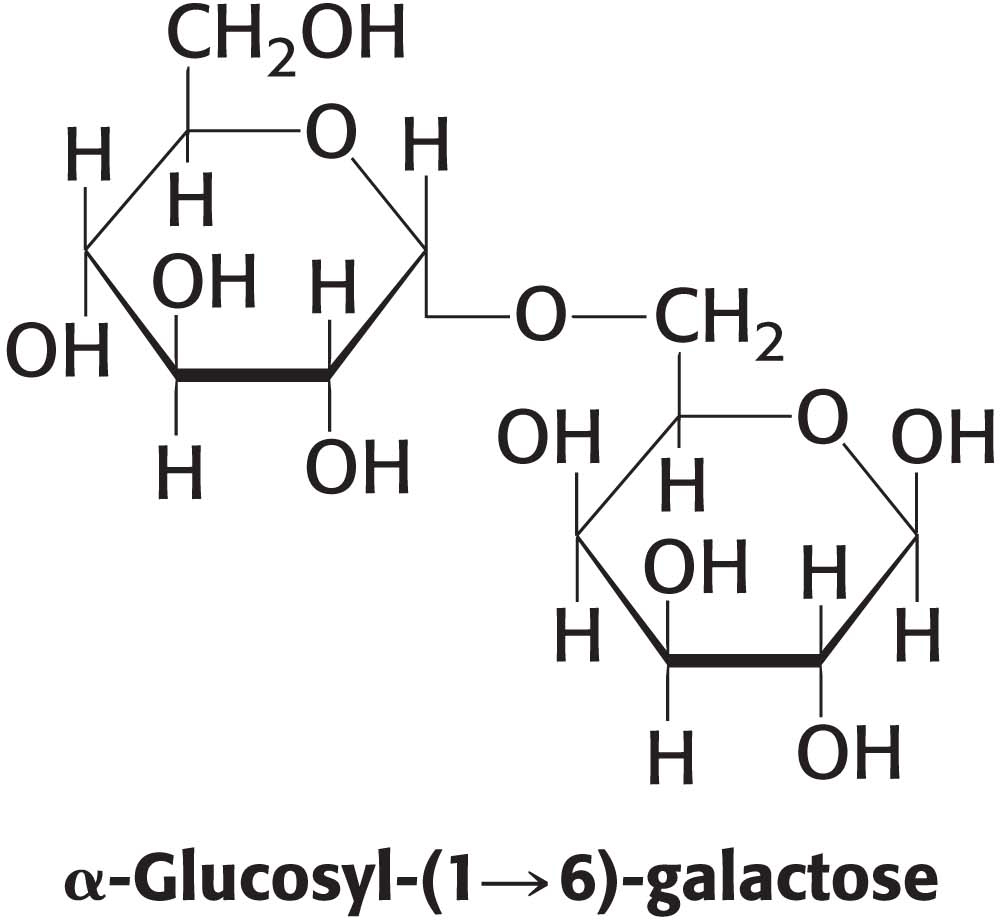
Draw the structure of the disaccharide α-glycosyl–

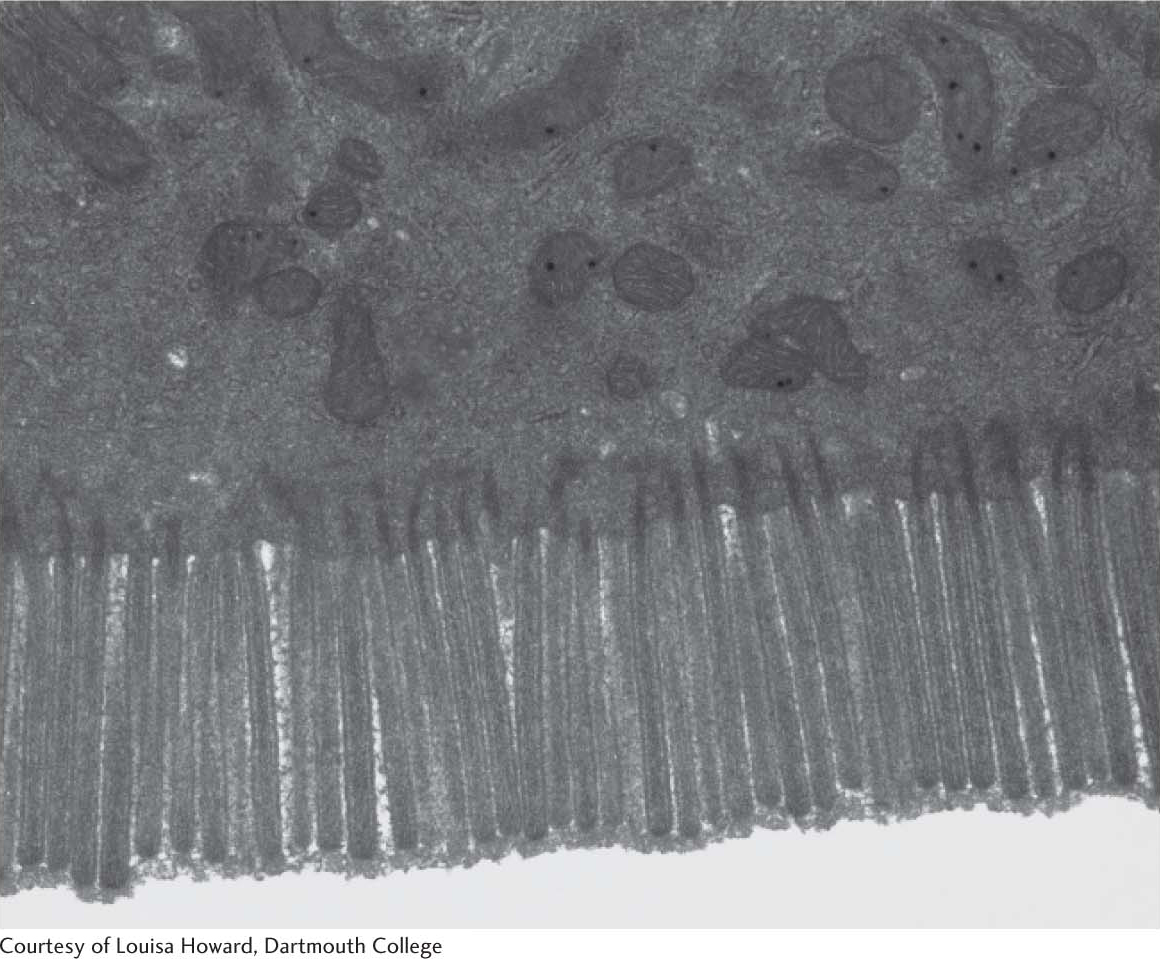
Cellulose, a Structural Component of Plants, Is Made of Chains of Glucose
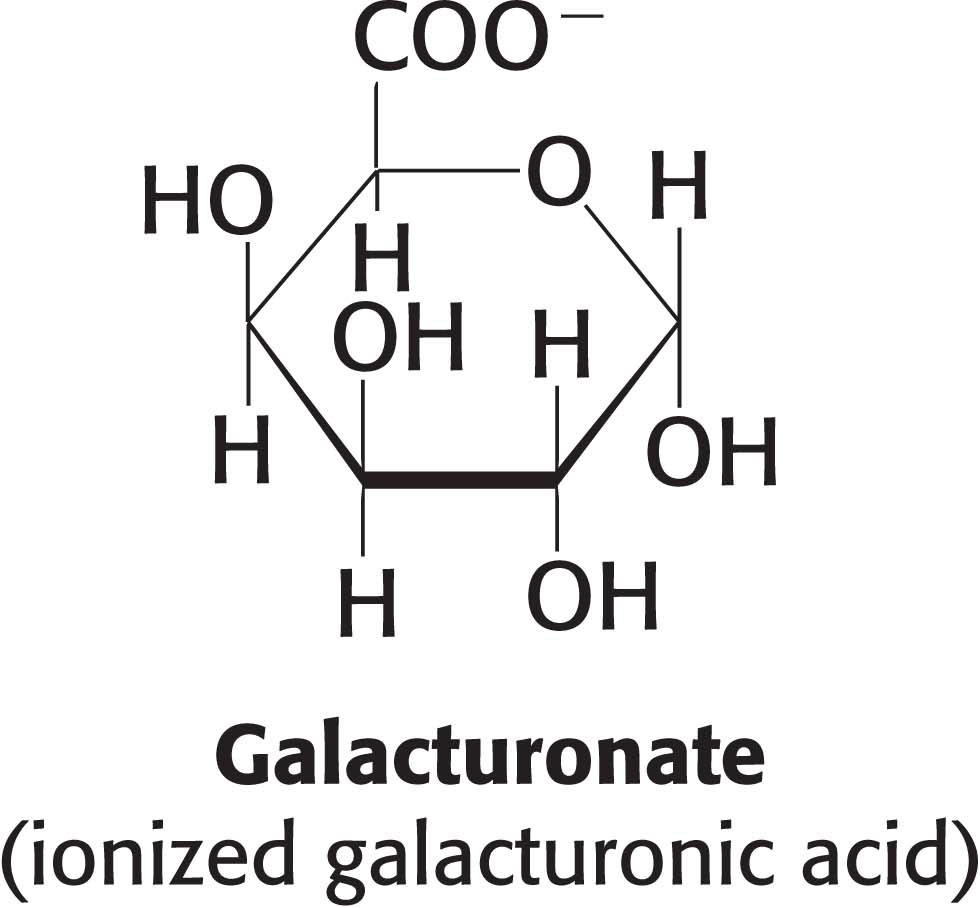
Cellulose, the other major polysaccharide of glucose found in plants, serves a structural rather than a nutritional role as an important component of the plant cell wall. Cellulose is among the most abundant organic compounds in the biosphere. Some 1015 kg of cellulose is synthesized and degraded on Earth each year, an amount 1000 times as great as the combined weight of the human race. Cellulose is an unbranched polymer of glucose residues joined by β-1,4 linkages, in contrast with the α-1,4 linkage seen in starch and glycogen. This simple difference in stereochemistry yields two molecules with very different properties and biological functions. The β configuration allows cellulose to form very long, straight chains. Fibrils are formed by parallel chains that interact with one another through hydrogen bonds, generating a rigid, supportive structure. The straight chains formed by β linkages are optimal for the construction of fibers having a high tensile strength. The α-1,4 linkages in glycogen and starch produce a very different molecular architecture: a hollow helix is formed instead of a straight chain (Figure 10.14). The hollow helix formed by α linkages is well suited to the formation of a more compact, accessible store of sugar. Although mammals lack cellulases and therefore cannot digest wood and vegetable fibers, cellulose and other plant fibers are still an important constituent of the mammalian diet as a component of dietary fiber. Soluble fiber such as pectin (polygalacturonate) slows the movement of food through the gastrointestinal tract, allowing improved digestion and the absorption of nutrients. Insoluble fibers, such as cellulose, increase the rate at which digestion products pass through the large intestine. This increase in rate can minimize exposure to toxins in the diet.