
10.4 Lectins Are Specific Carbohydrate-Binding Proteins
The diversity and complexity of the carbohydrate units and the variety of ways in which they can be joined in oligosaccharides and polysaccharides suggest that they are functionally important. Nature does not construct complex patterns when simple ones suffice. So why all this intricacy and diversity? It is now clear that these carbohydrate structures are the recognition sites for a special class of proteins. Such proteins, termed glycan-
Lectins Promote Interactions Between Cells
Cell–
We have already been introduced to a lectin obliquely. Recall that, in I-
 CLINICAL INSIGHT
CLINICAL INSIGHTLectins Facilitate Embryonic Development
One class of lectins consists of proteins termed selectins, which bind immune-
 CLINICAL INSIGHT
CLINICAL INSIGHTInfluenza Virus Binds to Sialic Acid Residues
Many pathogens gain entry into specific host cells by adhering to cell-
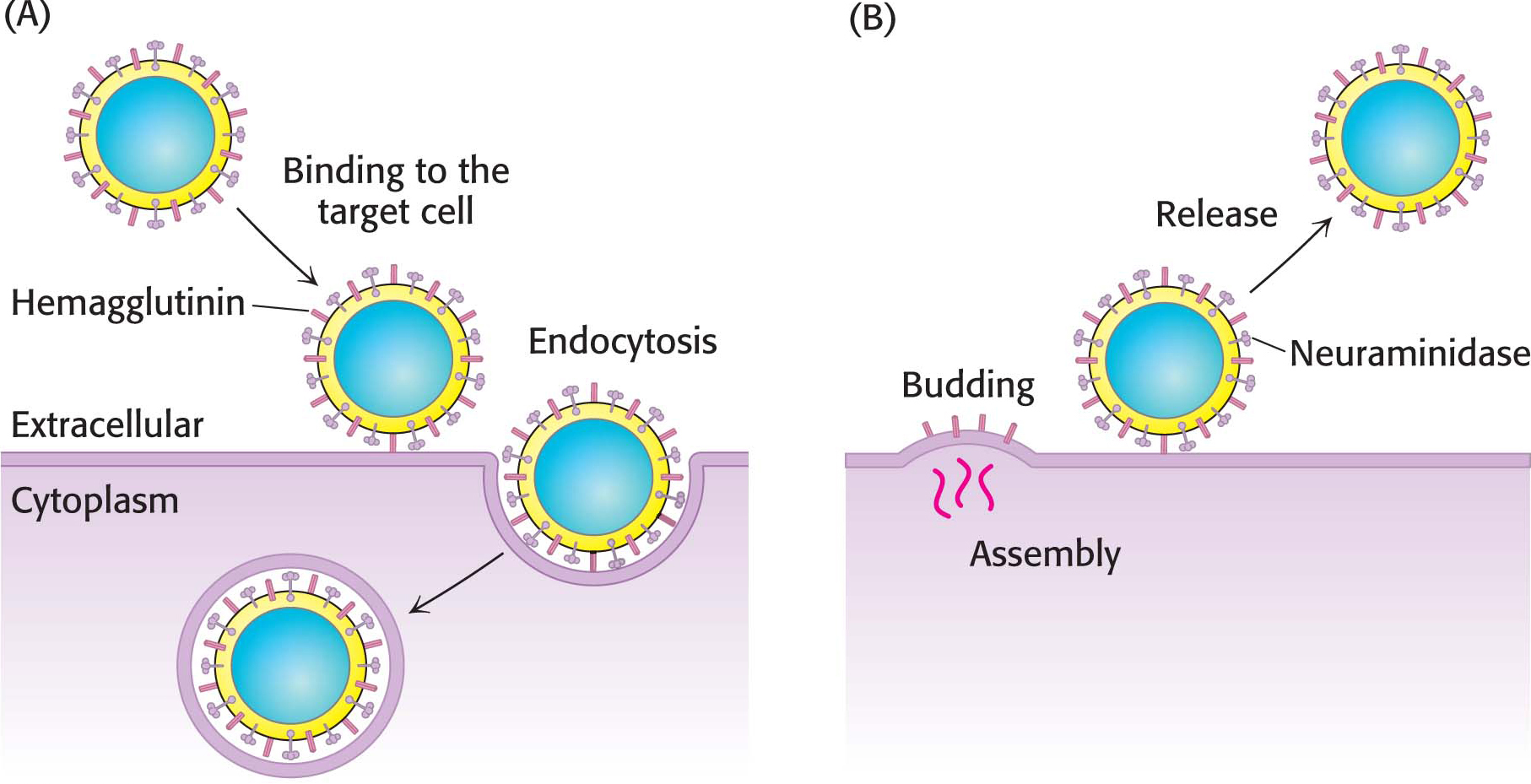
After binding to hemagglutinin, the virus is engulfed by the cell and begins to replicate. To exit the cell, a process essentially the reverse of viral entry occurs (Figure 10.27B). Viral assembly results in the budding of the viral particle from the cell. Upon complete assembly, the viral particle is still attached to sialic acid residues of the cell membrane by hemagglutinin on the surface of the new virions. Another viral protein, neuraminidase (sialidase), cleaves the glycosidic bonds between the sialic acid residues and the rest of the cellular glycoprotein, freeing the virus to infect new cells, spreading the infection throughout the respiratory tract. Inhibitors of this enzyme such as oseltamivir (Tamiflu) and zanamivir (Relenza) are important anti-
Viral hemagglutinin’s carbohydrate-