PROBLEMS
Question 10.1
1. Word origin. Account for the origin of the term carbohydrate. ✓ 1
Question 10.2
2. Diversity. How many different oligosaccharides can be made by linking one glucose, one mannose, and one galactose residue? Assume that each sugar is in its pyranose form. Compare this number with the number of tripeptides that can be made from three different amino acids. ✓ 1
Question 10.3
3. They go together like a horse and carriage. Match each term with its description.
Enantiomers Cellulose Lectins Glycosyltransferases Epimers Starch Carbohydrates Proteoglycan Mucoprotein Glycogen | Carbohydrate- Has the molecular formula of (CH2O)n The most abundant organic molecule in the biosphere The storage form of glucose in animals Enzymes that synthesize oligosaccharides The storage form of glucose in plants Stereoisomers that are mirror images of each other Glycoprotein containing glycosaminoglycans Monosaccharides that differ at a single asymmetric carbon atom N- |
Question 10.4
4. Couples. Indicate whether each of the following pairs of sugars consists of anomers, epimers, or an aldose–
(a) d-glyceraldehyde and dihydroxyacetone
(b) d-glucose and d-mannose
(c) d-glucose and d-fructose
(d) α-d-glucose and β-d-glucose
(e) d-ribose and d-ribulose
(f) d-galactose and d-glucose
Question 10.5
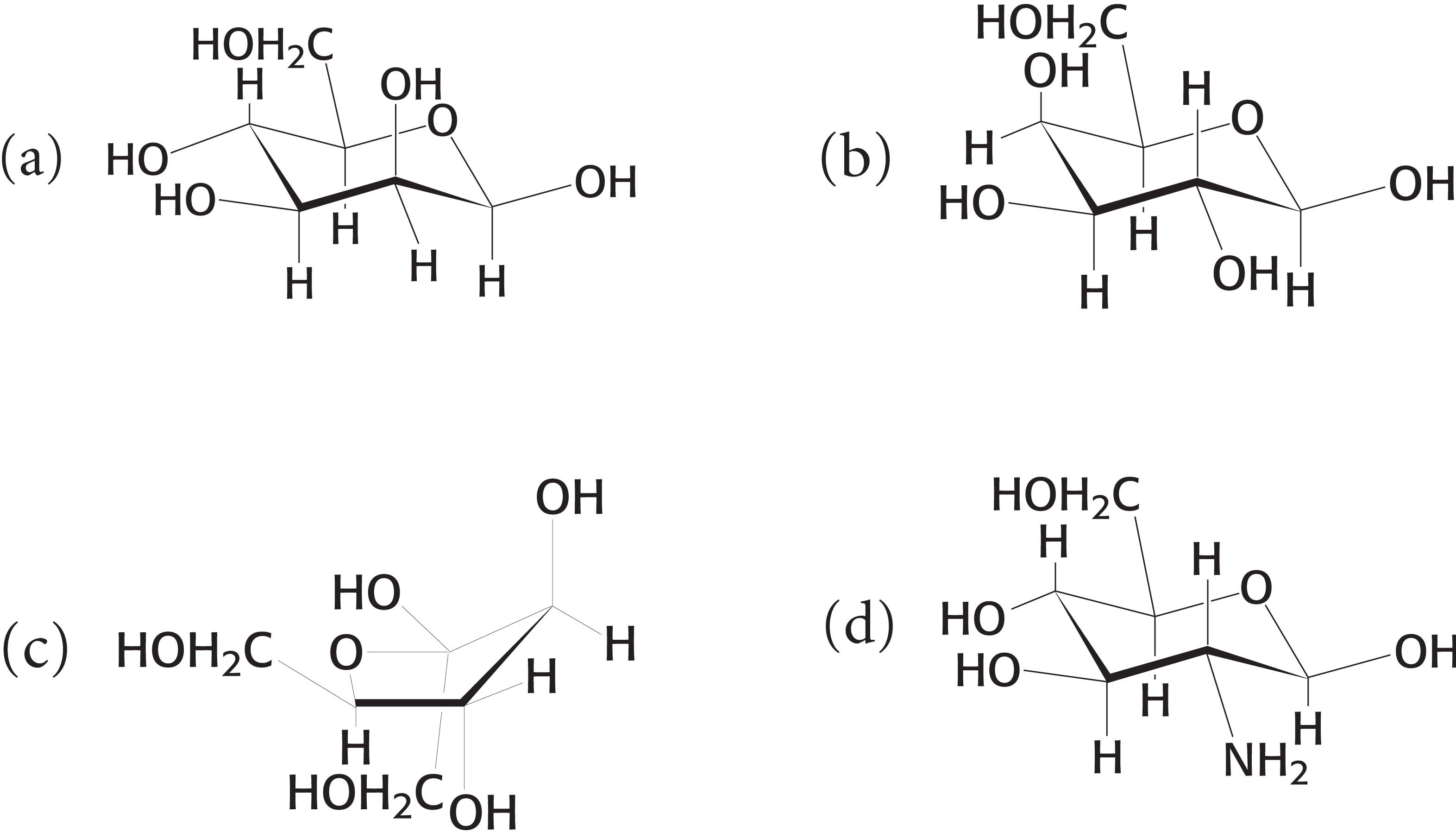
5. Carbons and carbonyls. To which classes of sugars do the monosaccharides shown here belong? ✓ 1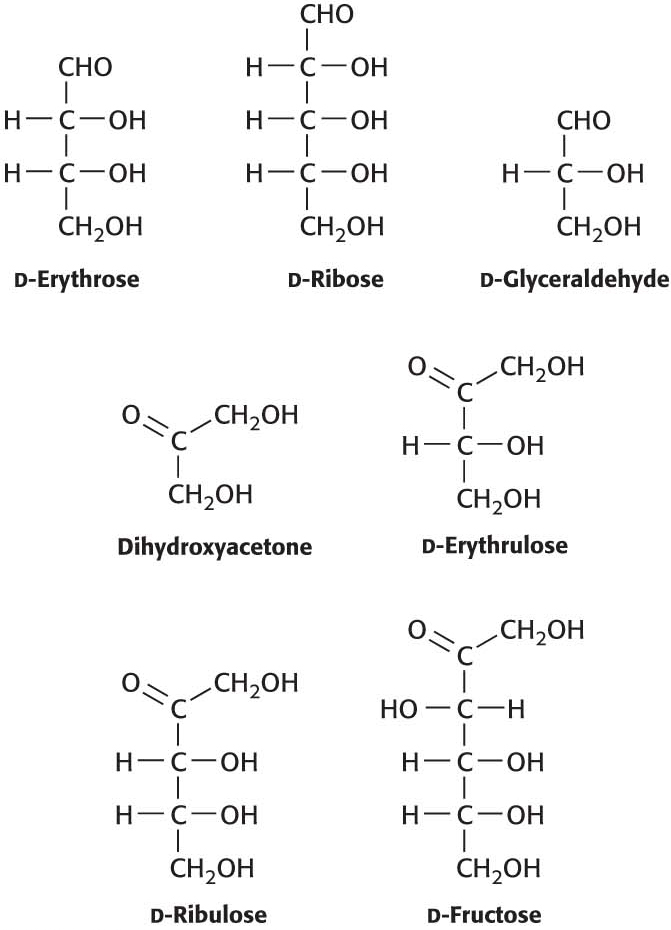
Question 10.6
6. Chemical cousins. Although an aldose with four asymmetric carbon atoms is capable of forming 16 diastereoisomers, only 8 of the isomers are commonly observed, including glucose. They are listed after the structure of d-glucose with their structural relation to glucose. Using the structure of glucose as a reference, draw the structures. ✓ 1
d-Allose: Epimeric at C-
d-Altrose: Isomeric at C-
d-Mannose: Epimeric at C-
d-Gulose: Isomeric at C-
d-Idose: Isomeric at C-
d-Galactose: Epimeric at C-
d-Talose: Isomeric at C-
Question 10.7
7. Telltale marker. Glucose reacts slowly with hemoglobin and other proteins to form covalent compounds. Why is glucose reactive? What is the nature of the product formed? ✓ 1
Question 10.8
8. A lost property. Glucose and fructose are reducing sugars. Sucrose, or table sugar, is a disaccharide consisting of both fructose and glucose. Is sucrose a reducing sugar? Explain. ✓ 1
Question 10.9
9. Oxygen source. Does the oxygen atom attached to C-
Question 10.11
11. Cellular glue. A trisaccharide unit of a cell-
Question 10.12
12. Component parts. Raffinose, a minor constituent in sugar beets, is a trisaccharide. ✓ 2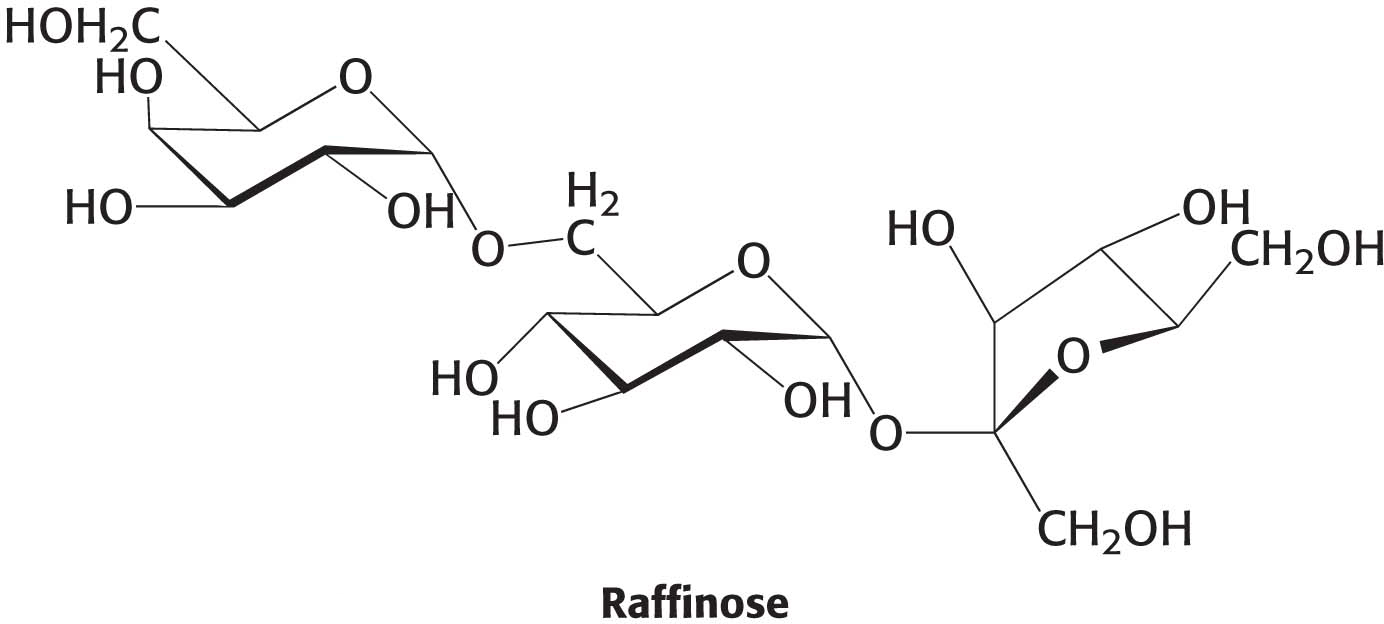
(a) Is raffinose a reducing sugar? Explain.
(b) What are the monosaccharides that compose raffinose?
(c) β-Galactosidase is an enzyme that will remove galactose residues from an oligosaccharide. What are the products of β-galactosidase treatment of raffinose?
Question 10.13
13. Anomeric differences. α-d-Mannose is a sweet-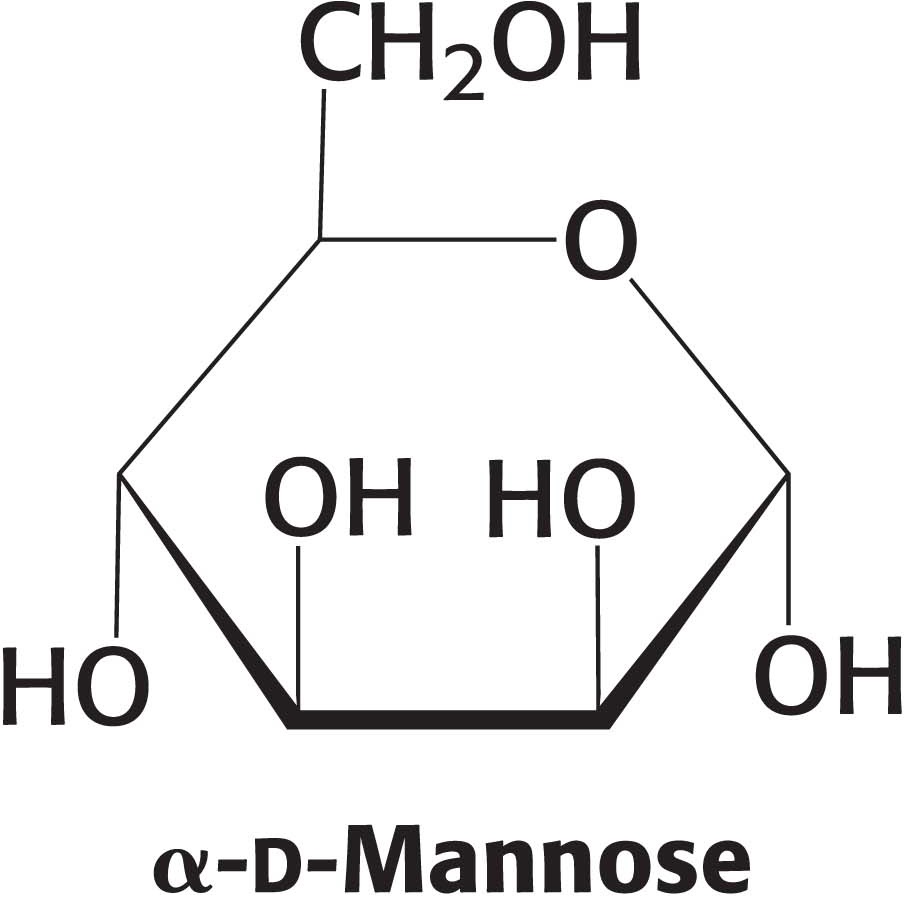
Question 10.14
14. A taste of honey. Fructose in its β-d-pyranose form accounts for the powerful sweetness of honey. The β-d-fu-
Question 10.15
15. Making ends meet. (a) Compare the number of reducing ends to nonreducing ends in a molecule of glycogen. (b) As we will see in Chapter 24, glycogen is an important fuel-
Question 10.16
16. Meat and potatoes. Compare the structures of glycogen and starch. ✓ 1
Question 10.17
17. Straight or with a twist? Account for the different structures of glycogen and cellulose. ✓ 1
Question 10.18
18. Sweet proteins. List the key classes of glycoprotein, their defining characteristics, and their biological functions. ✓ 2
Question 10.19
19. Life extender. What is the function of the carbohydrate moiety that is attached to EPO? ✓ 2
Question 10.20
20. Carbohydrates—not just for breakfast anymore. Differentiate between a glycoprotein and a lectin. ✓ 2
Question 10.21
21. Cushioning. What is the role of the glycosaminoglycan in the cushioning provided by cartilage? ✓ 2
Question 10.22
22. Locks and keys. What does the fact that all organisms contain lectins suggest about the role of carbohydrates? ✓ 2
Question 10.23
23. Undelivered mail. Not returned to sender. I-
Question 10.24
24. Carbohydrates and proteomics. Suppose that a protein contains six potential N-linked glycosylation sites. How many possible proteins can be generated, depending on which of these sites is actually glycosylated? Do not include the effects of diversity within the carbohydrate added. ✓ 2
Question 10.25
25. Many possibilities. Why are polysaccharides considered information-
Chapter Integration Problem
Question 10.26
26. Stereospecificity. Sucrose, a major product of photosynthesis in green leaves, is synthesized by a battery of enzymes. The substrates for sucrose synthesis, d-glucose and d-fructose, are a mixture of α and β anomers as well as acyclic compounds in solution. Nonetheless, sucrose consists of α-d-glucose linked by its C-
Data Interpretation Problem
Question 10.27
27. Sore joints. A contributing factor to the development of arthritis is the inappropriate proteolytic destruction of the aggrecan component of cartilage by the proteolytic enzyme aggre canase. The immune-
[Data from M. A. Pratta et al. J. Biol. Chem. 278:45539–
(a) Aggrecan degradation was measured by the release of glycosaminoglycan. What is the rational for this assay?
(b) Why might glycosaminoglycan release not indicate aggrecan degradation?
(c) What is the purpose of the control—
(d) What is the effect of adding IL-
(e) What is the response when an aggrecanase inhibitor is added in addition to IL-
(f) Why is there some aggrecan destruction in the control with the passage of time?
Challenge Problems
Question 10.28
28. Mutarotation. The specific rotations of the α and β anomers of d-glucose are +112 degrees and +18.7 degrees, respectively. Specific rotation, [α]D, is defined as the observed rotation of light of wavelength 589 nm (the d line of a sodium lamp) passing through 10 cm of a 1 g ml−1 solution of a sample. When a crystalline sample of α-d-glucopyranose is dissolved in water, the specific rotation decreases from 112 degrees to an equilibrium value of 52.7 degrees. On the basis of this result, what are the proportions of the α and β anomers at equilibrium? Assume that the concentration of the open-
Question 10.29
29. Periodate cleavage. Compounds containing hydroxyl groups on adjacent carbon atoms undergo carbon–
Question 10.30
30. Mapping the molecule. Each of the hydroxyl groups of glucose can be methylated with reagents such as dimethyl-
Selected Readings for this chapter can be found online at www.whfreeman.com/