
11.1 Fatty Acids Are a Main Source of Fuel
✓ 3 Describe the key chemical properties of fatty acids.
Fats, or fatty acids, are chains of hydrogen-
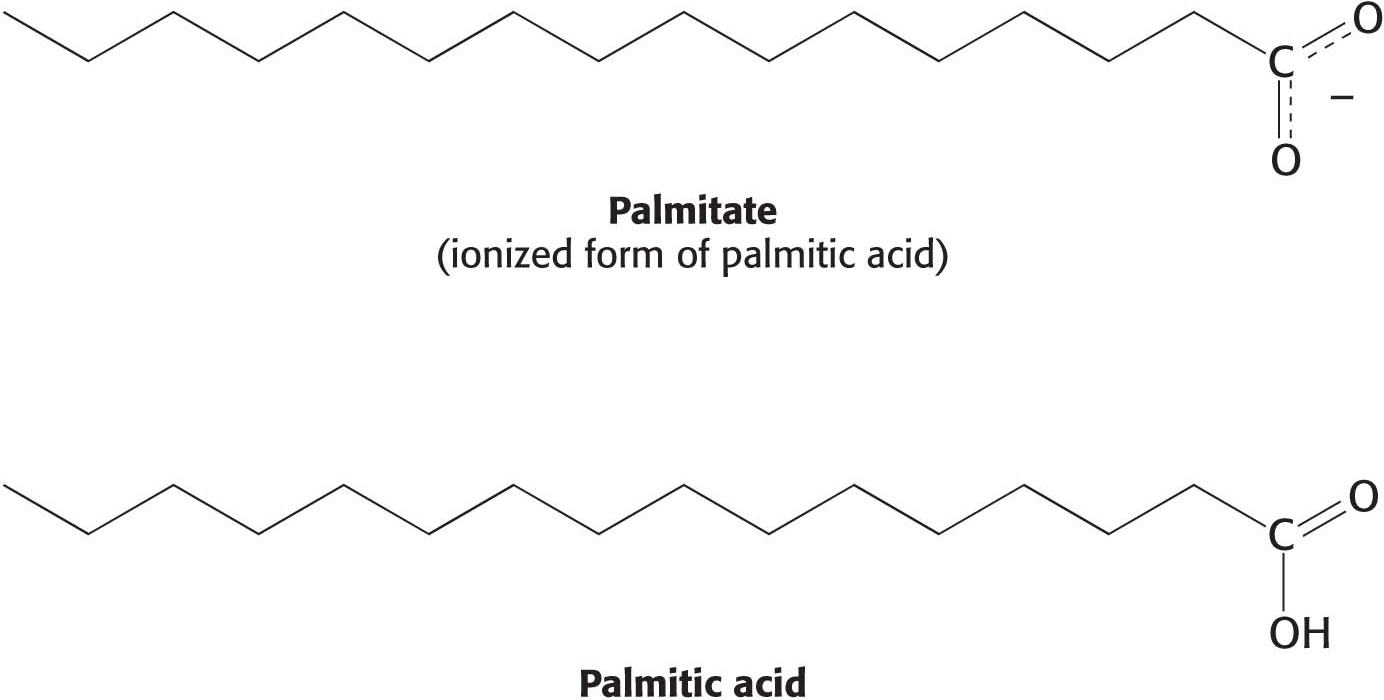
Fats have common names and systematic names. Although we will use the common names for fatty acids, familiarity with the systematic names is important because their use is sometimes required to prevent confusion. A fatty acid’s systematic name is derived from the name of its parent hydrocarbon by the substitution of oic for the final e. For example, the C18 saturated fatty acid known familiarly as stearic acid is systematically called octadecanoic acid because the parent hydrocarbon is octadecane: octadec-for the 18 carbon atoms, and –ane because it is composed completely of single bonds. Fatty acids composed of single bonds only are called saturated fatty acids, because every carbon atom is attached to four other atoms. Fatty acids with one or more double or triple bonds are called unsaturated fatty acids. Oleic acid, a C18 fatty acid with one double bond, is called octadecenoic acid; whereas linoleic acid, with two double bonds, is octadecadienoic acid; and linolenic acid, with three double bonds, is octadecatrienoic acid. The composition and structure of a fatty acid can also be designated by numbers. The notation 18:0 denotes a C18 fatty acid with no double bonds, whereas 18:2 signifies that there are two double bonds. The structure of the ionized forms of two common fatty acids—
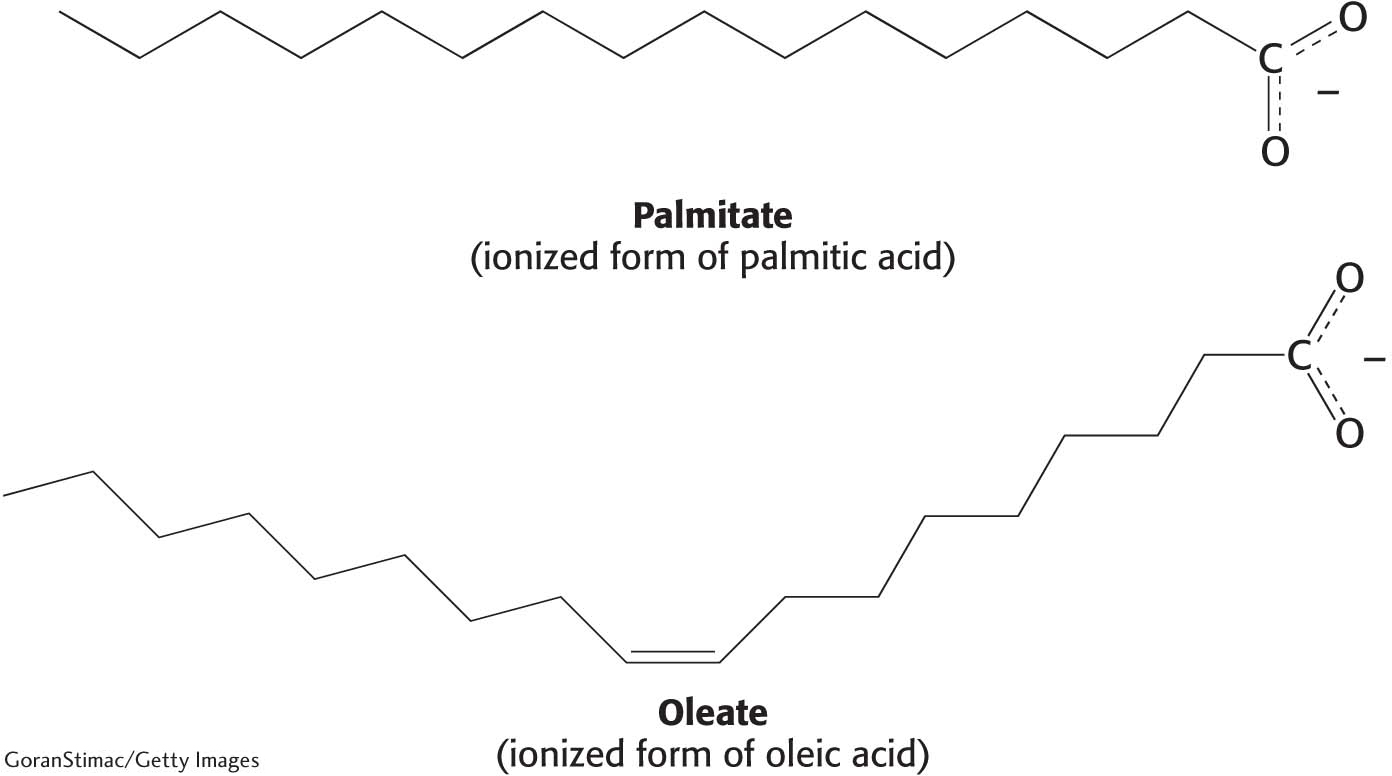
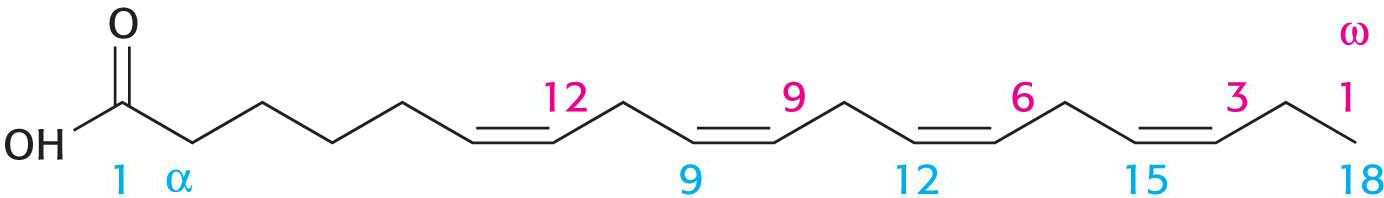
When considering fatty acids, we often need to distinguish the individual carbon atoms. Fatty acid carbon atoms are usually numbered starting at the carboxyl terminus but can also be numbered starting at the terminal carbon atom, as shown in the margin. Carbon atoms 2 and 3 are often referred to as α and β, respectively. The last carbon atom in the chain, which is almost always a methyl carbon atom, is called the ω-carbon atom (ω is the Greek symbol for omega, the last letter of the Greek alphabet). The position of a double bond is represented by the symbol Δ followed by a superscript number. For example, cis-Δ9 means that there is a cis double bond between carbon atoms 9 and 10; trans-Δ2 means that there is a trans double bond between carbon atoms 2 and 3. Cis and trans designate the relative positions of substituents on either side of the double bond. Just like amino acids, fatty acids are ionized at physiological pH, and so it is preferable to refer to them according to their carboxylate form: for example, palmitate rather than palmitic acid.
Fatty Acids Vary in Chain Length and Degree of Unsaturation
Fatty acids in biological systems usually contain an even number of carbon atoms, typically between 14 and 24 (Table 11.1), with the 16-
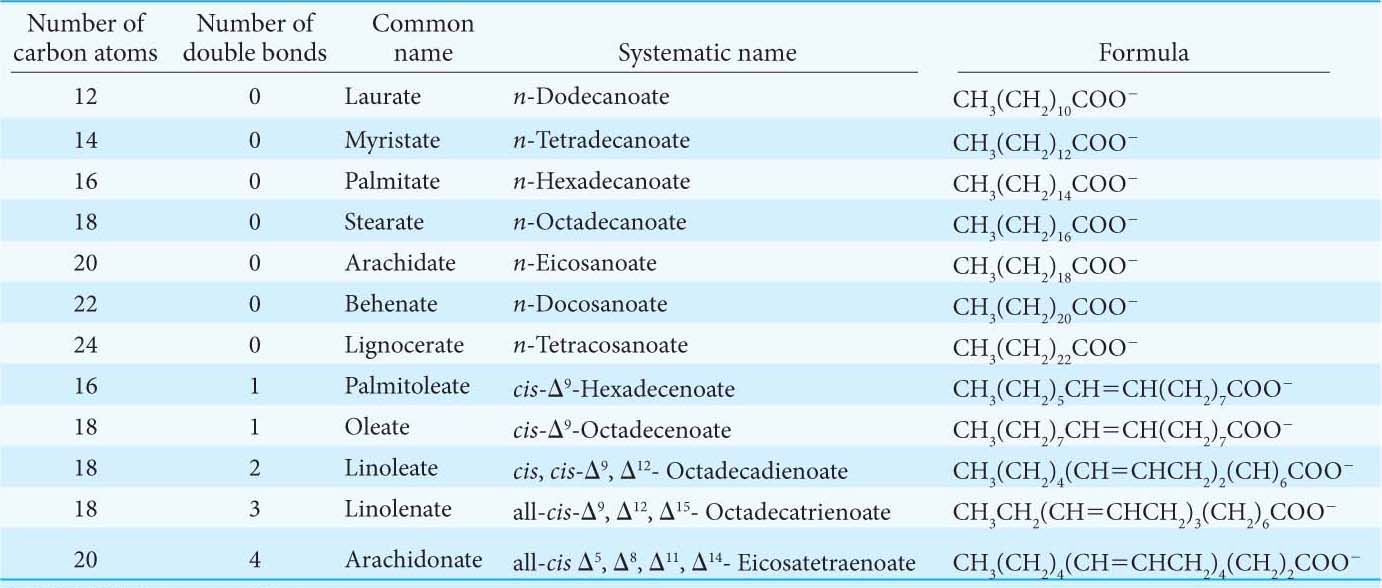
The properties of fatty acids and of lipids derived from them are markedly dependent on chain length and degree of saturation. Unsaturated fatty acids have lower melting points than those of saturated fatty acids of the same length. For example, the melting point of stearic acid is 69.6°C, whereas that of oleic acid (which contains one cis double bond) is 13.4°C. The melting points of polyunsaturated fatty acids of the C18 series are even lower. The presence of a cis double bond introduces a kink in the fatty acid and makes tight packing between the chains impossible. The lack of tight packing limits the van der Waals interactions between chains, lowering the melting temperature.
QUICK QUIZ 1
What factors determine the melting point of fatty acids?
Chain length and the degree of cis unsaturation.
Chain length also affects the melting point, as illustrated by the fact that the melting temperature of palmitic acid (C16) is 6.5 degrees lower than that of stearic acid (C18). The fat that accumulates in the pan as bacon is fried is composed primarily of saturated fatty acids and solidifies soon after the burner is turned off. Olive oil, on the other hand, is composed of high concentrations of oleic acid and some polyunsaturated fatty acids and remains liquid at room temperature. The variability of melting points is not merely an arcane chemical insight. Melting temperatures of fatty acids are key elements in the control of the fluidity of cell membranes, and the proper degree of fluidity is essential for membrane function (Chapter 12). Thus, short chain length and cis unsaturation enhance the fluidity of fatty acids and of their derivatives.
The Degree and Type of Unsaturation Are Important to Health
Although fats are crucial biochemicals, too much saturated and trans-
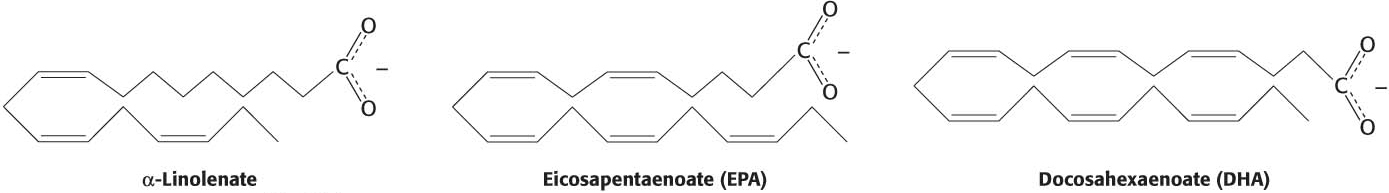
These fatty acids are precursors to important hormones. However, the nature of their protective effect against heart disease is not known. Studies show that ω-3 fatty acids, especially those derived from marine organisms, can prevent sudden death from a heart attack, reduce triacylglycerols in the blood, lower blood pressure, and prevent thrombosis by slightly inhibiting blood clotting. Revealing how these molecules exert their effects is an active area of biochemical research.