
11.3 There Are Three Common Types of Membrane Lipids
Thus far, we have considered only one type of complex lipid—
Phospholipids Are the Major Class of Membrane Lipids
Phospholipids are abundant in all biological membranes. A phospholipid molecule is constructed from four components: one or more fatty acids, a platform to which the fatty acids are attached, a phosphate, and an alcohol attached to the phosphate.
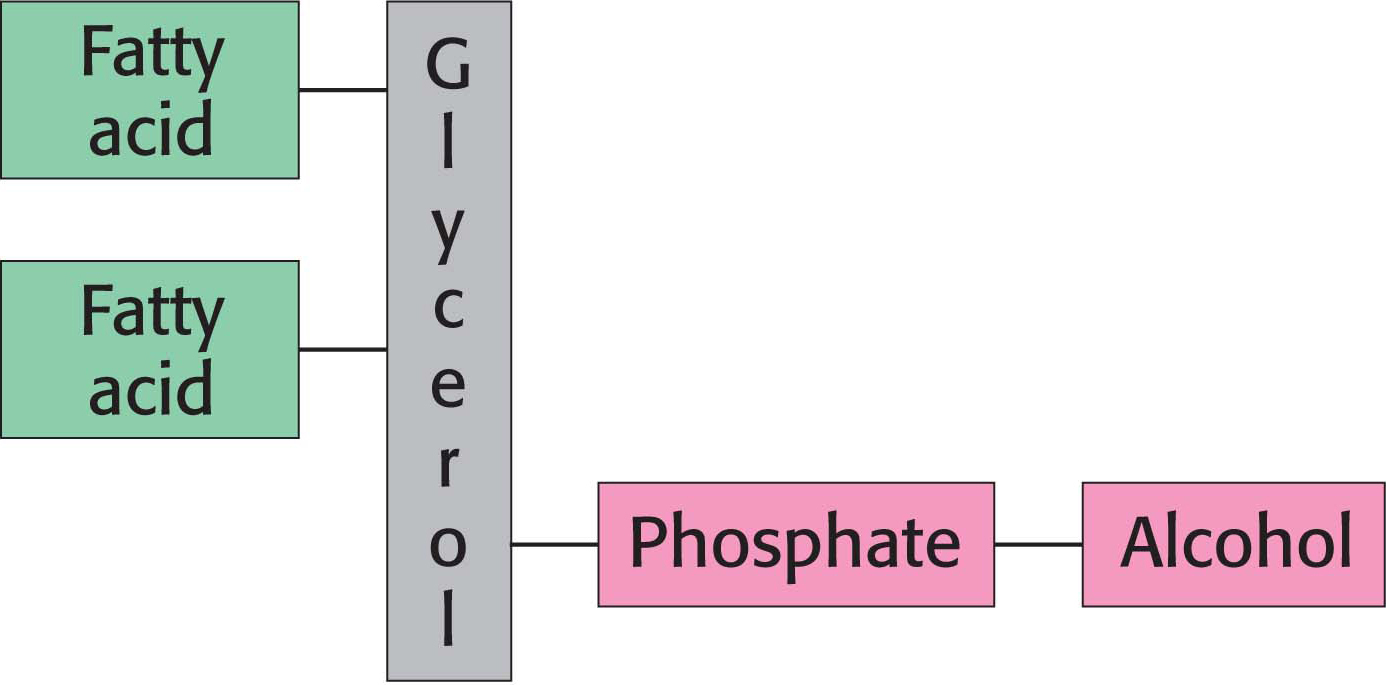
The platform on which phospholipids are built may be glycerol, a three-
In phosphoglycerides, the hydroxyl groups at C-
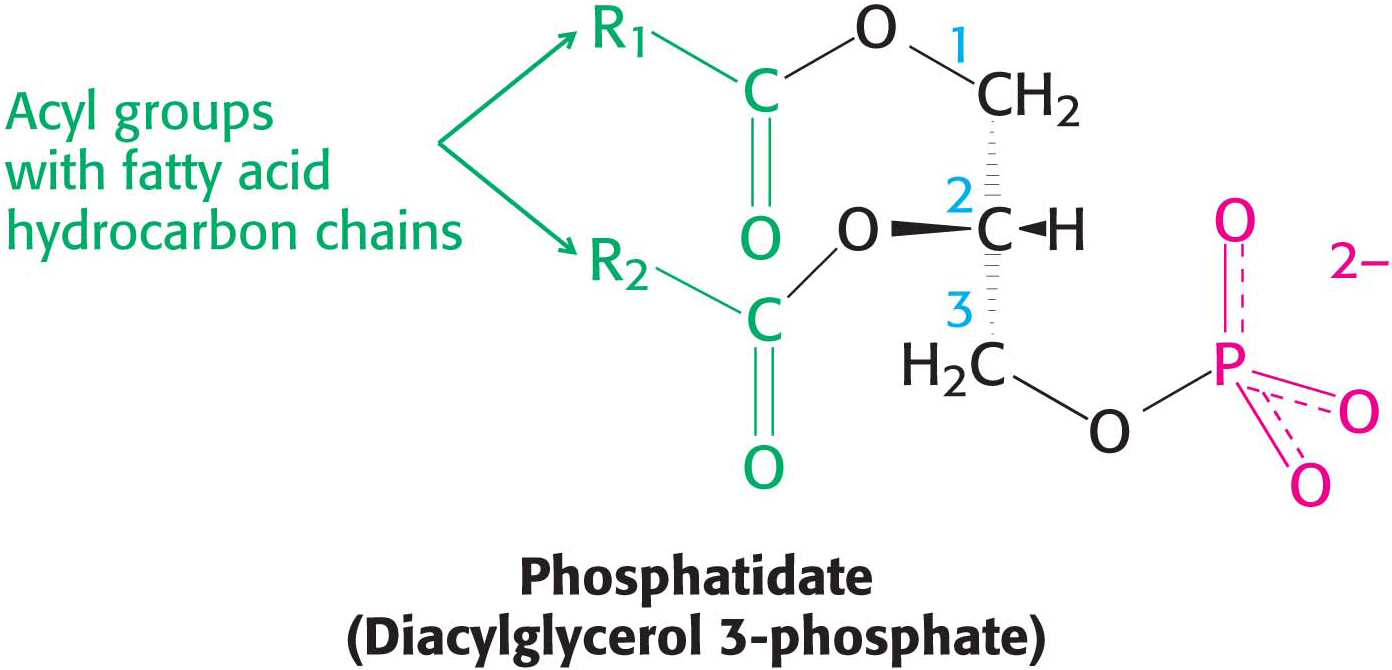
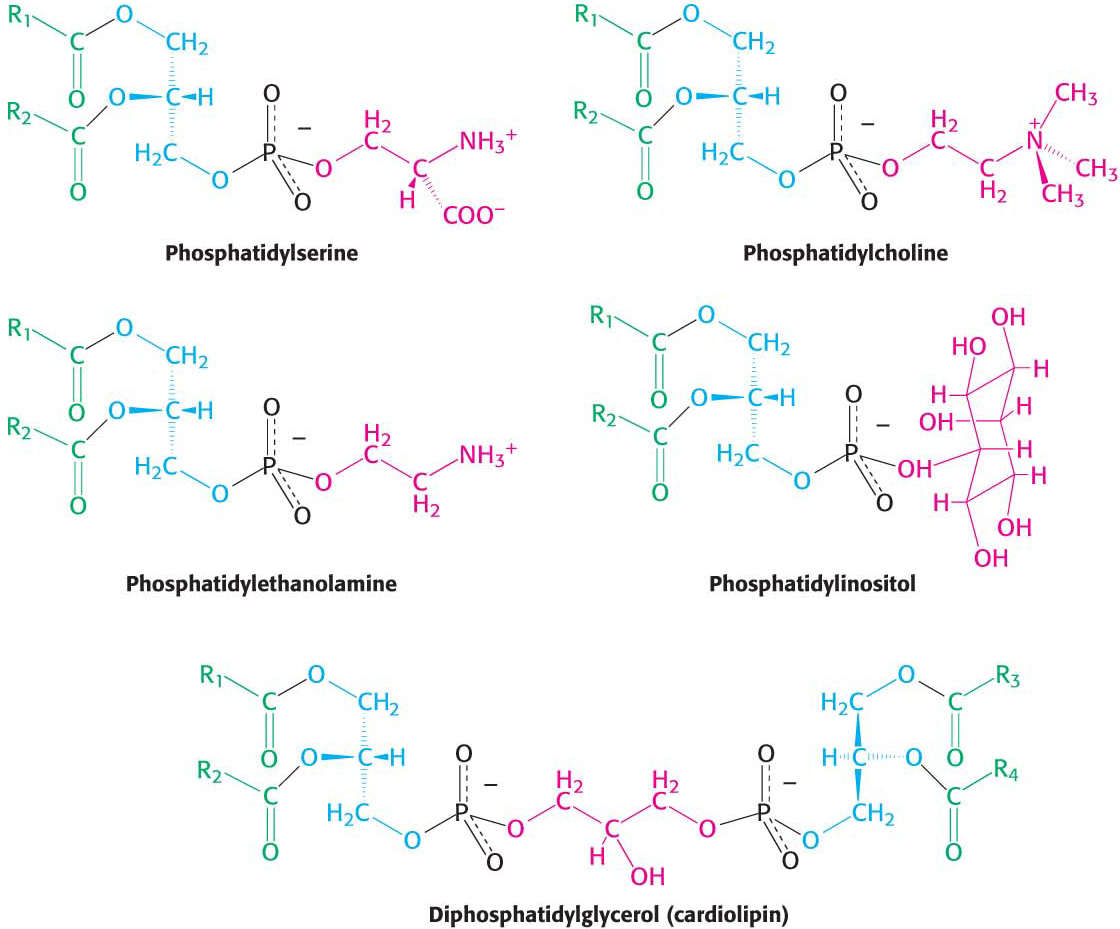
The major phosphoglycerides are derived from phosphatidate by the formation of an ester linkage between the phosphoryl group of phosphatidate and the hydroxyl group of one of several alcohols. The common alcohol moieties of phosphoglycerides are the amino acid serine, ethanolamine, choline, glycerol, and inositol. The structural formulas of phosphatidylcholine and the other principal phosphoglycerides are given in Figure 11.7.
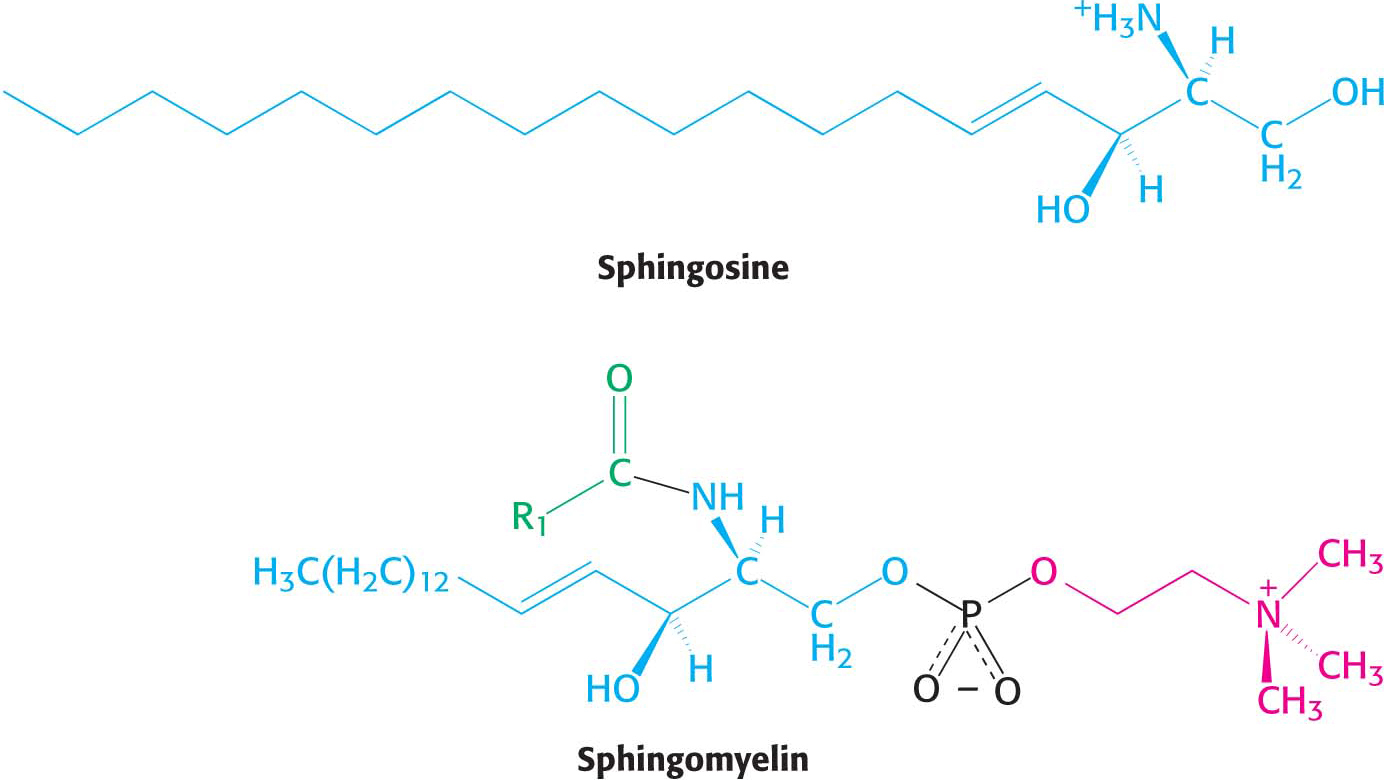
Phospholipids built on a sphingosine backbone are called sphingolipids. Sphingosine is an amino alcohol that contains a long, unsaturated hydrocarbon chain (Figure 11.8). Sphingomyelin is a common sphingolipid found in membranes. In sphingomyelin, the amino group of the sphingosine backbone is linked to a fatty acid by an amide bond. In addition, the primary hydroxyl group of sphingosine is attached to phosphorylcholine through an ester linkage. Sphingomyelin is found in the plasma membrane of many cells but is especially rich in the myelin sheath of nerve cells.
DID YOU KNOW?
Niemann–
Membrane Lipids Can Include Carbohydrates
Glycolipids, as their name implies, are sugar-
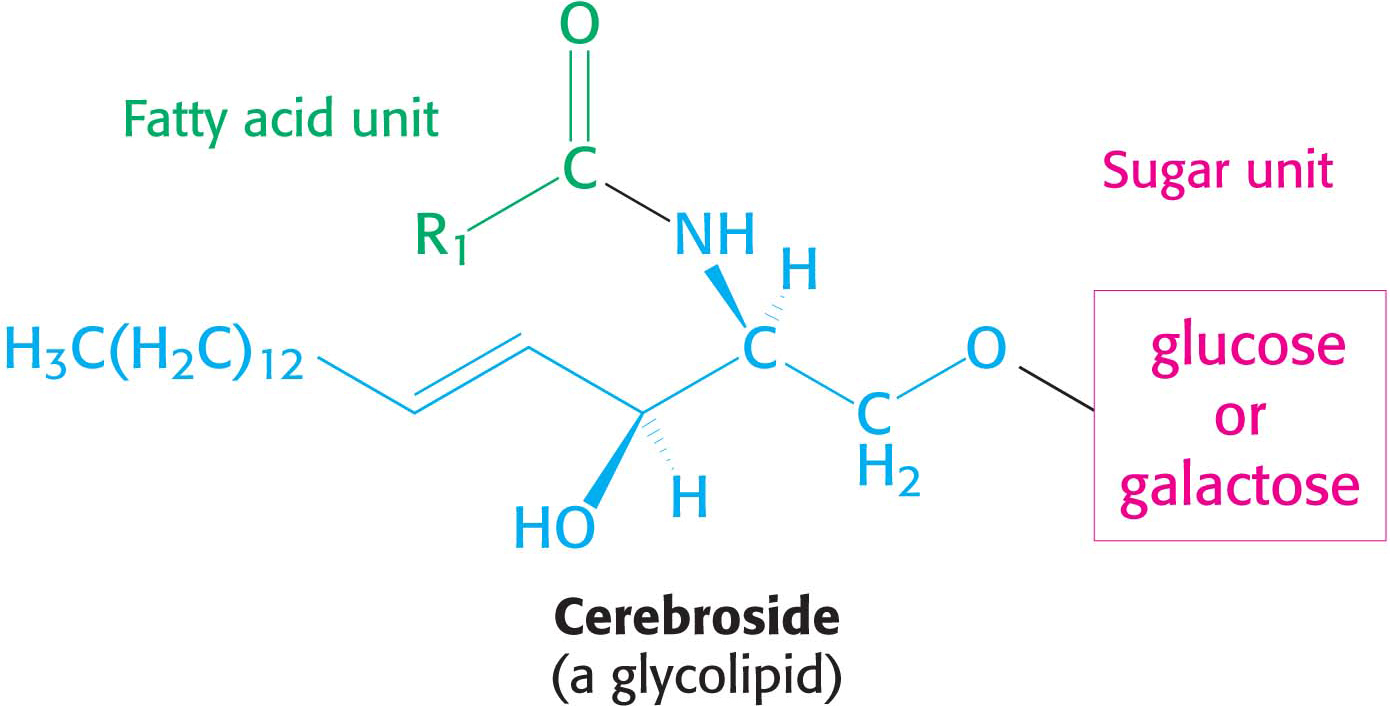
More complex glycolipids, such as gangliosides, contain a branched chain of as many as seven sugar residues. Glycolipids are oriented in an asymmetric fashion in membranes with the sugar residues always on the extracellular side of the membrane.
Steroids Are Lipids That Have a Variety of Roles
Steroids, the final class of lipids to be considered in this chapter, function as powerful hormones such as estradiol and testosterone, facilitate the digestion of lipids in the diet, and are key membrane constituents. Unlike the other classes of lipids, steroids exhibit a cyclical rather than linear structure. All steroids have a tetracyclic ring structure called the steroid nucleus. The steroid nucleus consists of three cyclohexane rings and a cyclopentane ring joined together.
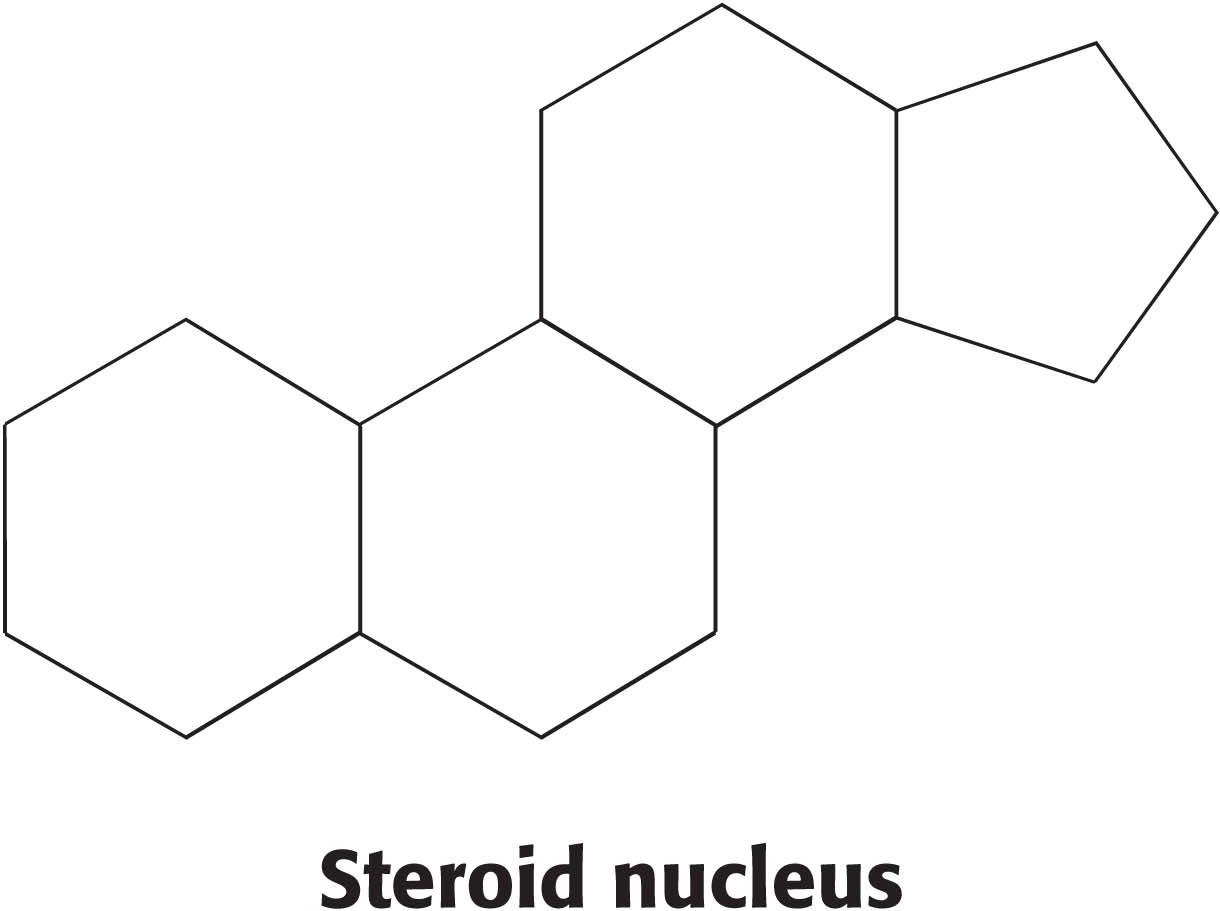
All biochemically important steroids are modified versions of this basic structure.
Cholesterol is the most common steroid, and the precursor to biochemically active steroids. It is a member of a class of molecules called sterol—
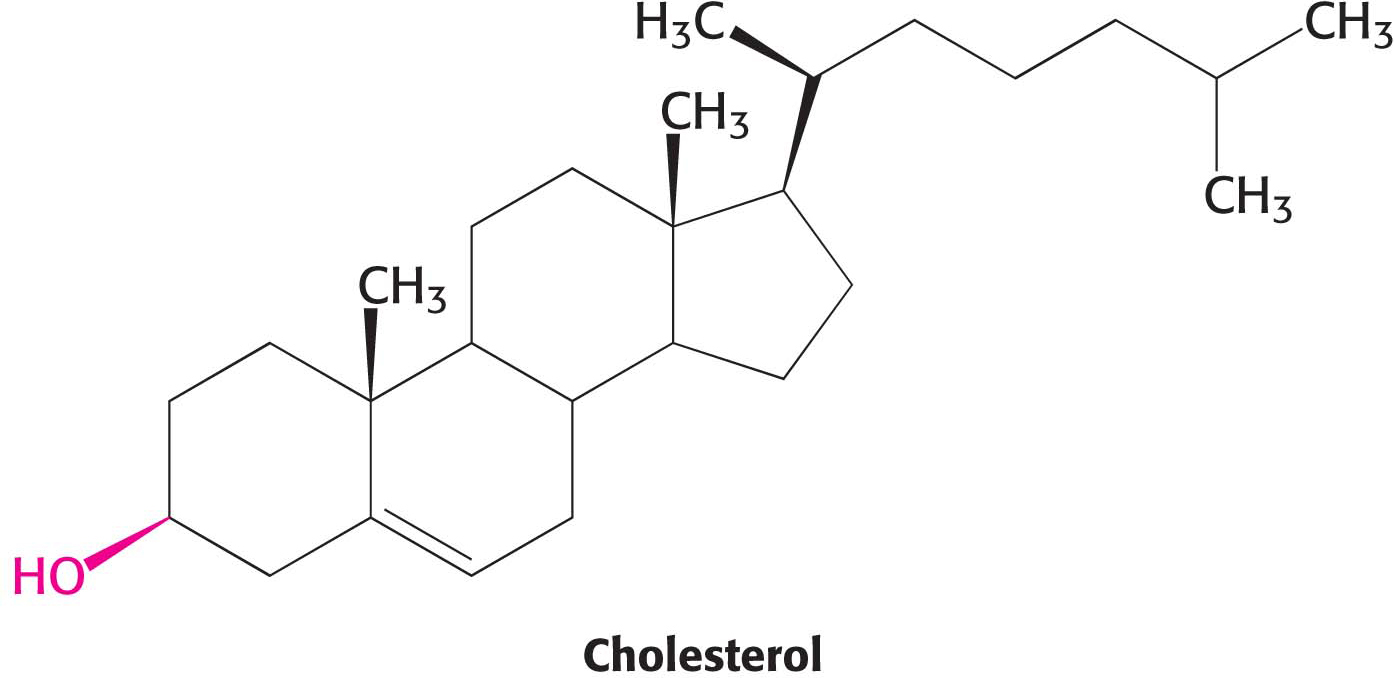
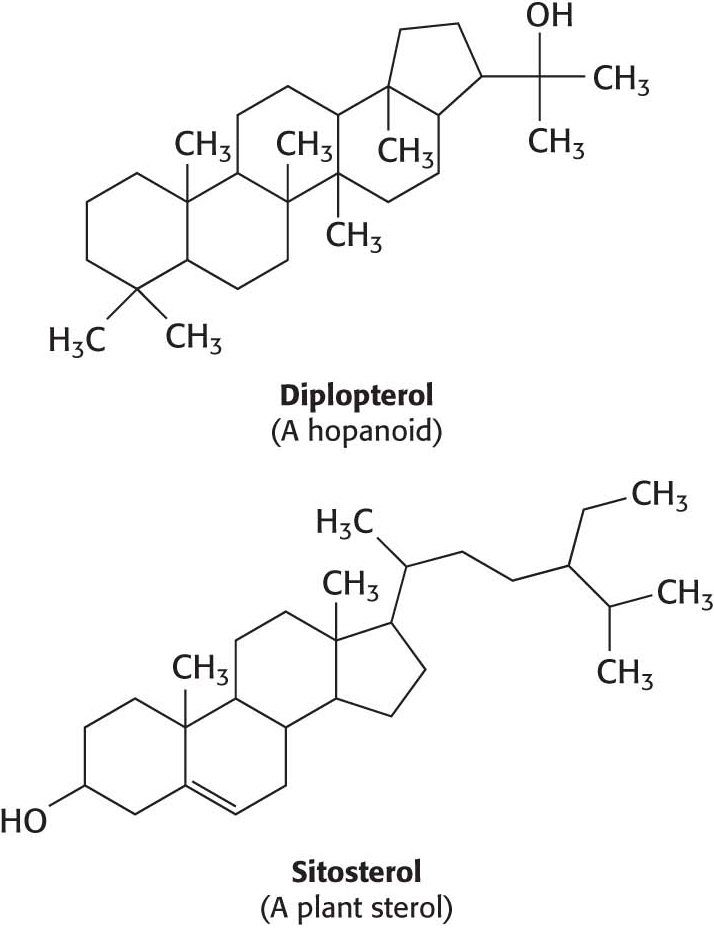
A hydrocarbon tail is linked to the steroid at one end, and a hydroxyl group is attached at the other end. As we will see in Chapter 12, cholesterol is important in maintaining proper membrane fluidity. Although cholesterol is absent from prokaryotes, all organisms require a similar molecule to moderate membrane fluidity. In prokaryotes, hopanoids play the role of cholesterol while various sterols serve the function in plants. Cholesterol is found in varying degrees in virtually all animal membranes. It constitutes almost 25% of the membrane lipids in certain nerve cells but is essentially absent from some intracellular membranes. Free cholesterol does not exist outside of membranes. Rather, it is esterified to a fatty acid for storage and transport.
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTMembranes of Extremophiles Are Built from Ether Lipids with Branched Chains
The membranes of archaea differ in composition from those of eukaryotes or bacteria in two important ways. These differences clearly relate to the hostile living conditions of many archaea (Figure 8.3). First, the fatty acid chains are joined to a glycerol backbone by ether rather than ester linkages. The ether linkage is more resistant to hydrolysis than the ester linkage is. Second, the alkyl chains are branched rather than linear. They are built up from repeats of a fully saturated five-
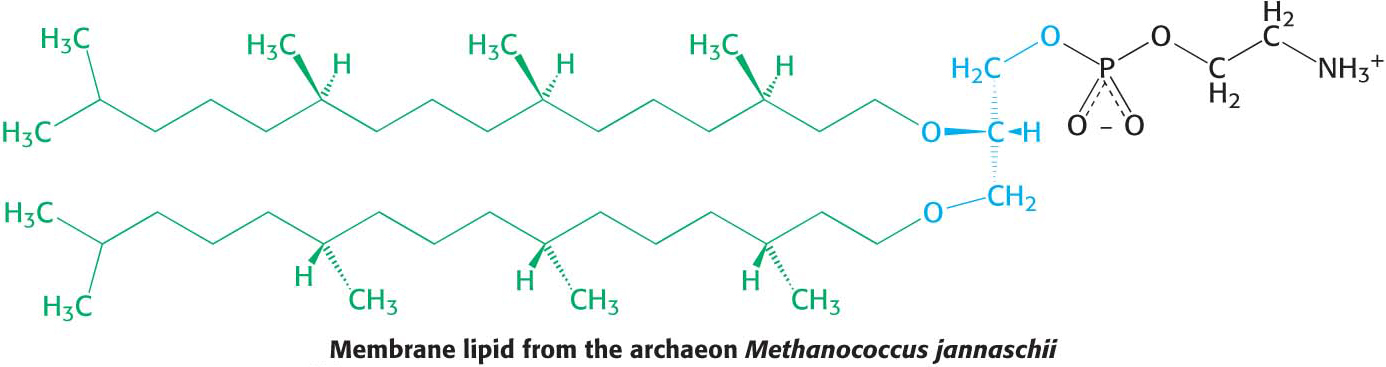
The ability of these lipids to resist hydrolysis and oxidation may help these organisms to withstand the extreme conditions, such as high temperature, low pH, or high salt concentration, under which some of these archaea grow.
Membrane Lipids Contain a Hydrophilic and a Hydrophobic Moiety
The repertoire of membrane lipids is extensive. However, these lipids possess a critical common structural theme: membrane lipids are amphipathic molecules (amphiphilic molecules) containing both a hydrophilic and a hydrophobic moiety.
QUICK QUIZ 2
What are the three major types of membrane lipids?
Phospholipids, sphingolipids (of which glycolipids are a subclass), and cholesterol.
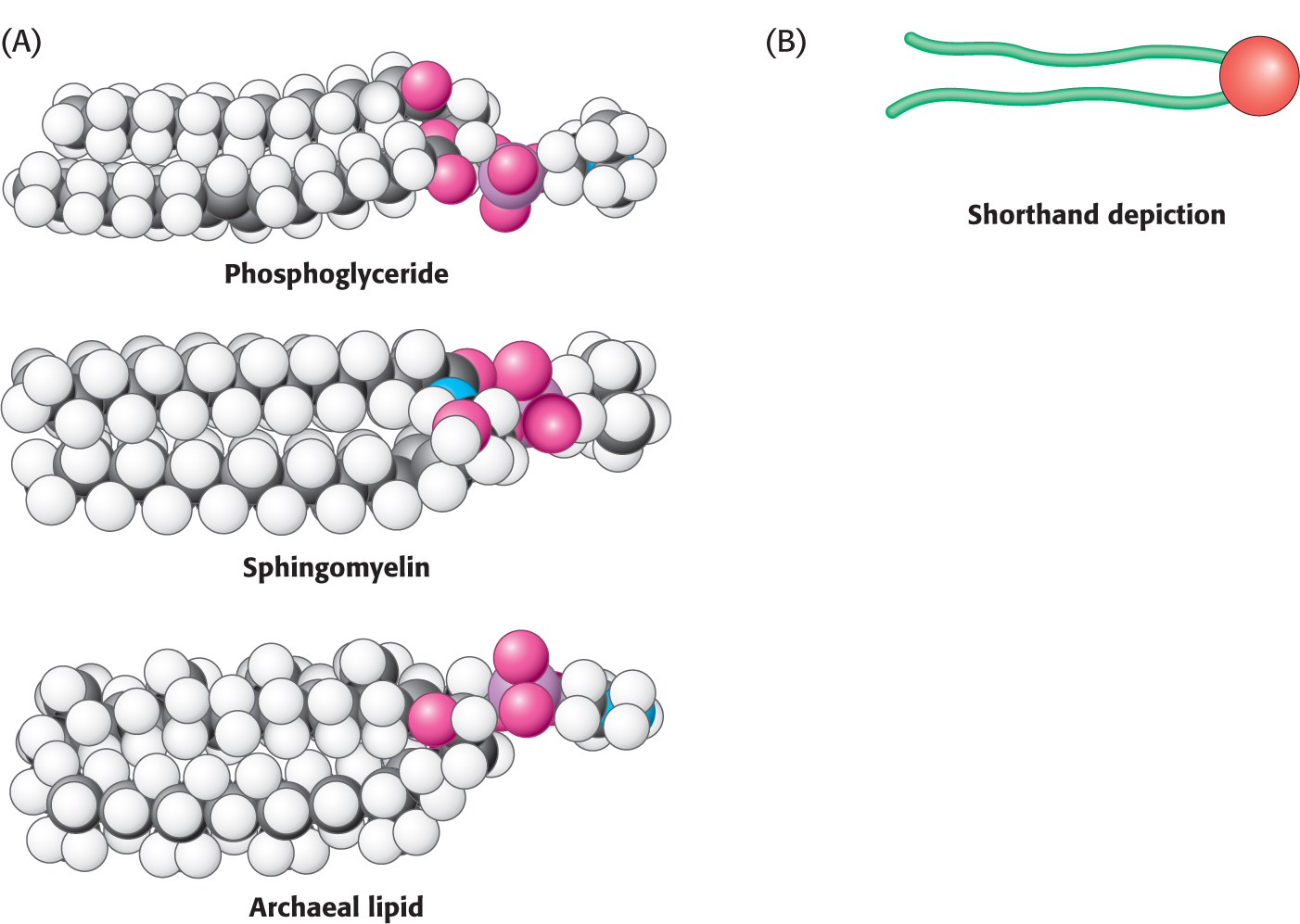
Let us look at a model of a phosphoglyceride, such as phosphatidylcholine. Its overall shape is roughly rectangular (Figure 11.9). The two hydrophobic fatty acid chains are approximately parallel to each other, whereas the hydrophilic phosphorylcholine moiety points in the opposite direction. Sphingomyelin has a similar conformation, as does the archaeal lipid depicted. Therefore, the following shorthand has been adopted to represent these membrane lipids: the hydrophilic unit, also called the polar head group, is represented by a circle, whereas the hydrocarbon tails are depicted by straight or wavy lines (Figure 11.9B).
Some Proteins Are Modified by the Covalent Attachment of Hydrophobic Groups
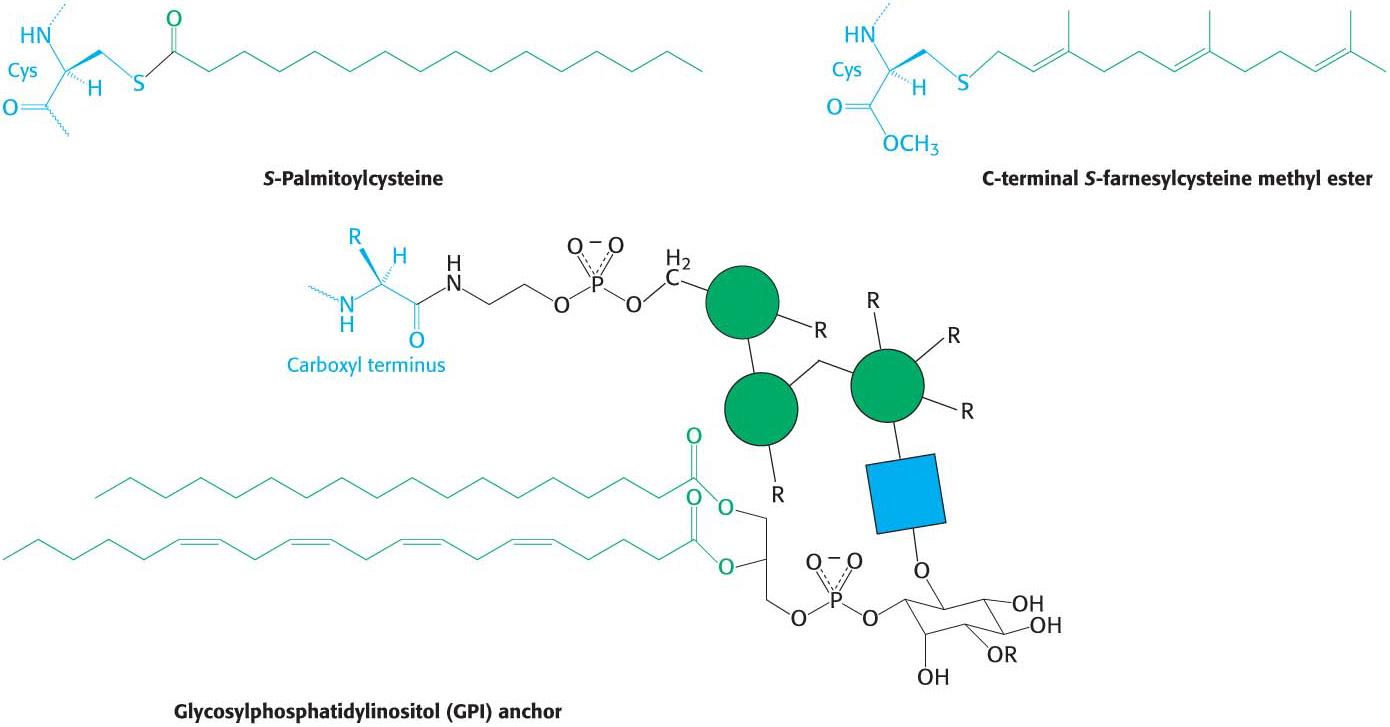
Just as carbohydrates are attached to proteins to modify the properties of proteins, lipids also can be attached to proteins to provide them with additional biochemical properties. Often, such attachments are necessary for a protein to associate with a hydrophobic environment such as a membrane. These hydrophobic attachments can insert into the hydrophobic interior of the membrane and localize the protein to the membrane surface. Such localization is required for protein function. Three such attachments are shown in Figure 11.10: (1) a palmitoyl group attached to a cysteine residue by a thioester bond, (2) a farnesyl group attached to a cysteine residue at the carboxyl terminus, and (3) a glycolipid structure termed a glycosylphosphatidylinositol (GPI) anchor attached to the carboxyl terminus.
 CLINICAL INSIGHT
CLINICAL INSIGHTPremature Aging Can Result from the Improper Attachment of a Hydrophobic Group to a Protein
Farnesyl is a hydrophobic group that is often attached to proteins, usually so that the protein is able to associate with a membrane (Figure 11.10). Inappropriate farnesylation has been shown to result in Hutchinson–
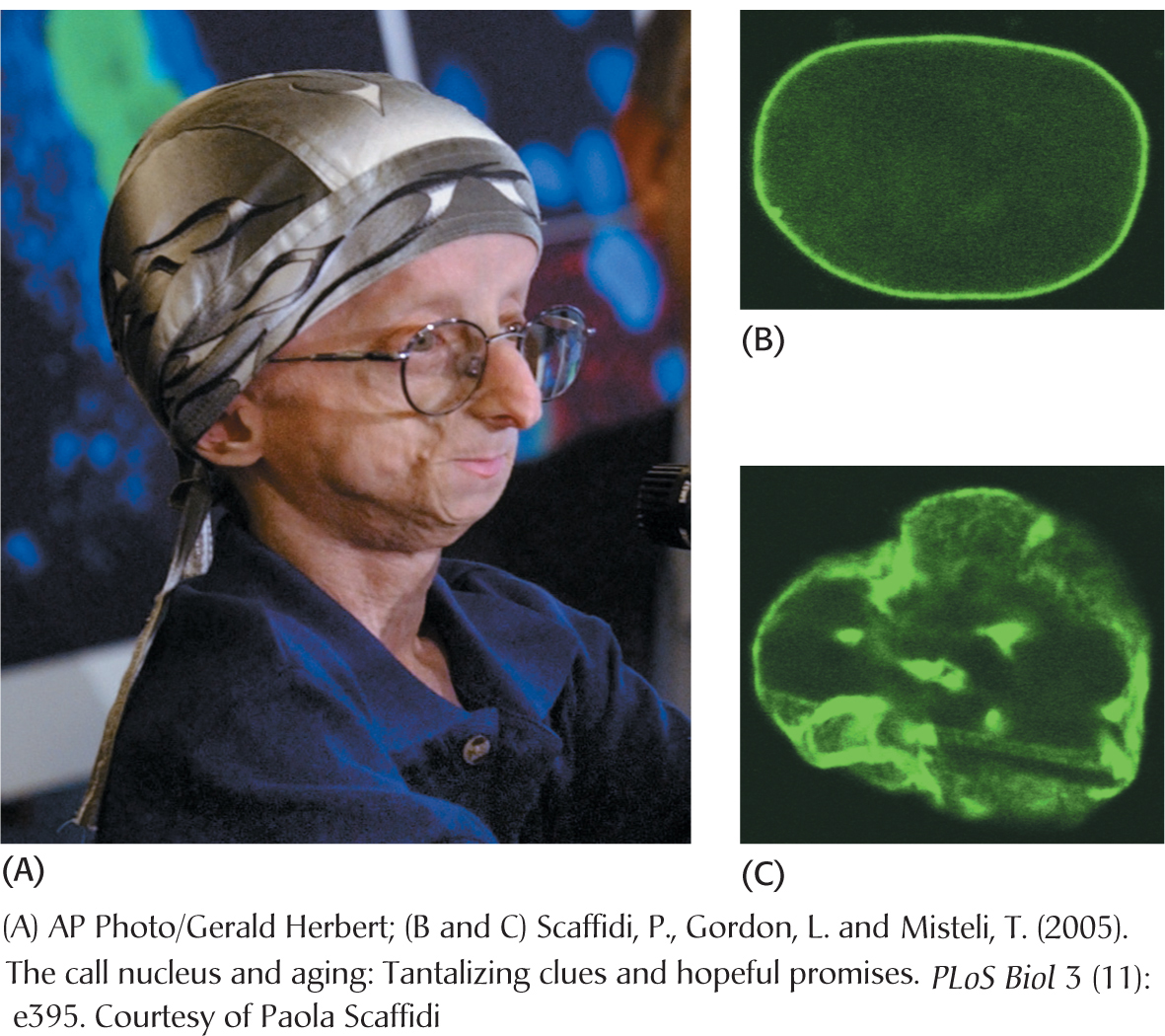
The cause of HGPS appears to be a mutation in the gene for the nuclear protein lamin, a protein that forms a scaffold for the nucleus and may take part in the regulation of gene expression. The folded polypeptide that will eventually become lamin is modified and processed many times before the mature protein is produced. One key processing event is the removal of a farnesyl group that had been added to the nascent protein earlier in processing. In HGPS patients, the farnesyl group is not removed, owing to a mutation in the lamin. The incorrectly processed lamin results in a deformed nucleus (Figure 11.11) and aberrant nuclear function that results in HGPS. Much research remains to determine precisely how the failure to remove the farnesyl group leads to such dramatic consequences.