PROBLEMS
Question 11.1
1. Definition. What are lipids? ✓ 3
Question 11.2
2. Like Lady and the Tramp. Match the term with the proper description. ✓ 4
Fatty acid Triacylglycerol Phospholipid Sphingosine Phosphoglyceride Sphingomyelin Glycolipid Cerebroside Galactoside Cholesterol | A complex alcohol backbone for membrane lipids The simplest glycolipid Phospholipid especially common in nerve cells Major class of membrane lipids Membrane lipids with a glycerol backbone Chains of hydrogen- Storage form of fatty acids Steroid- Complex glycolipids Derived from sphingosine and found in all membranes |
Question 11.3
3. Structure and name. Draw the structure of each of the following fatty acids, and give the structure its common name. ✓ 3
(a) n-Dodecanate
(b) cis-Δ9-Hexadecenoate
(c) cis, cis-Δ9, Δ12-Octadecadienoate
Question 11.4
4. Fluidity matters. Triacylglycerols are used for fuel storage in both plants and animals. The triacylglycerols from plants are often liquid at room temperature, whereas those from animals are solid. Suggest some chemical reasons for this difference. ✓ 3
Question 11.5
5. Contrast. Distinguish between phosphoglycerides and triacylglycerols. ✓ 4
Question 11.6
6. Compare. What structural features differentiate sphingolipids from phosphoglycerides? ✓ 4
Question 11.7
7. The head of the matter. What are some molecules that form the polar head group of phospholipids? ✓ 4
Question 11.8
8. Depict a lipid. Draw the structure of a triacylglycerol composed of equal amounts of palmitic acid, stearic acid, and oleic acid. ✓ 4
Question 11.9
9. Like finds like. What structural characteristic of lipids accounts for their solubility in organic solvents? ✓ 4
Question 11.10
10. Like finds like, again. Suppose that a small amount of phospholipid were exposed to an aqueous solution. What structure would the phospholipid molecules assume? What would be the driving force for the formation of this structure? ✓ 4
Question 11.11
11. Repetitive pattern. How does the structure of steroids differ from the structure of other lipids? ✓ 4
Question 11.12
12. Linkages. Platelet-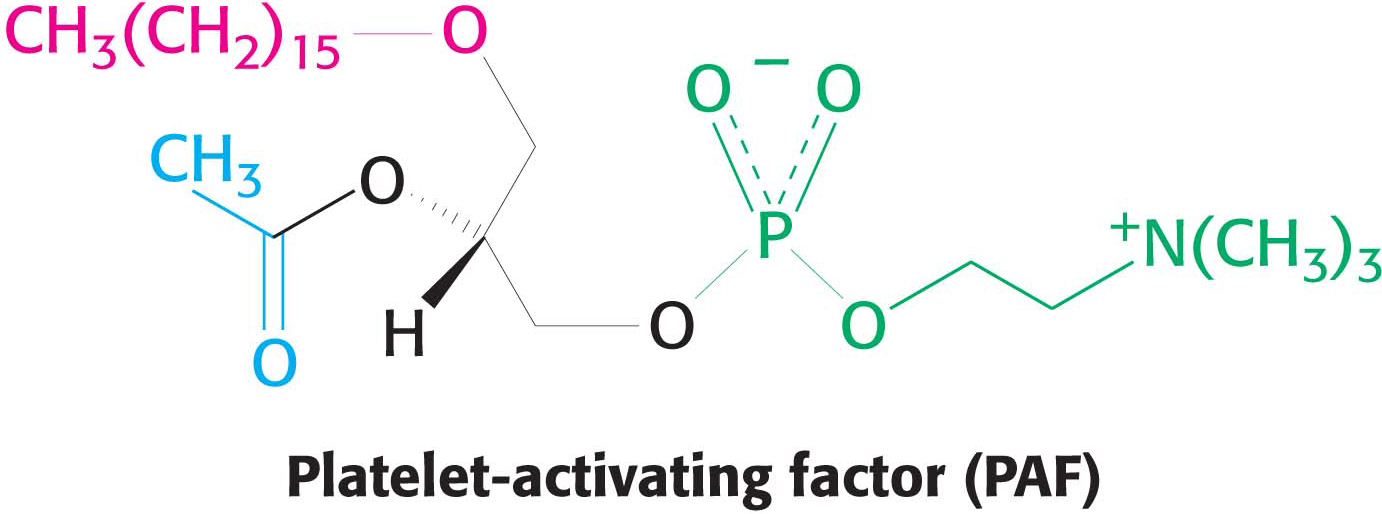
Question 11.13
13. Coming clean. Draw the structure of the sodium salt of stearic acid. How might it function to remove grease from your clothes or your hands? ✓ 3
Question 11.14
14. Hard-
Question 11.15
15. Efficiency matters. Why are lipids a more efficient storage form than glycogen? ✓ 4
Question 11.16
16. A sound diet. Small mammalian hibernators can withstand body temperatures of 0° to 5°C without injury. However, the body fats of most mammals have melting temperatures of approximately 25°C. Predict how the composition of the body fat of hibernators might differ from that of their nonhibernating cousins. ✓ 3
Challenge Problems
Question 11.17
17. Molecules and melting points. Match the fatty acid with the appropriate melting point. ✓ 3
CH3(CH2)18CO2H CH3(CH2)14CO2H CH3(CH2)10CO2H CH3(CH2)7CH=CH(CH2)7CO2H CH3(CH2)5CH=CH(CH2)7CO2H CH3(CH2)4CH=CHCH2CH=CH (CH2)7CO2H CH3(CH2)4(CH=CHCH2)4 (CH2)2CO2H | −49°C 76°C −5°C 63°C 0°C 45°C 13°C |
Question 11.18
18. Melting point 1. Explain why oleic acid (18 carbons, one cis bond) has a lower melting point than stearic acid, which has the same number of carbon atoms but is saturated. How would you expect the melting point of trans-oleic acid to compare with that of cis-oleic acid? ✓ 3
Question 11.19
19. Melting point 2. Explain why the melting point of palmitic acid (C16) is 6.5 degrees lower than that of stearic acid (C18). ✓ 3
Selected Readings for this chapter can be found online at www.whfreeman.com/