12.1 Phospholipids and Glycolipids Form Bimolecular Sheets
✓ 1 Identify the energetic force that powers the formation of membranes.
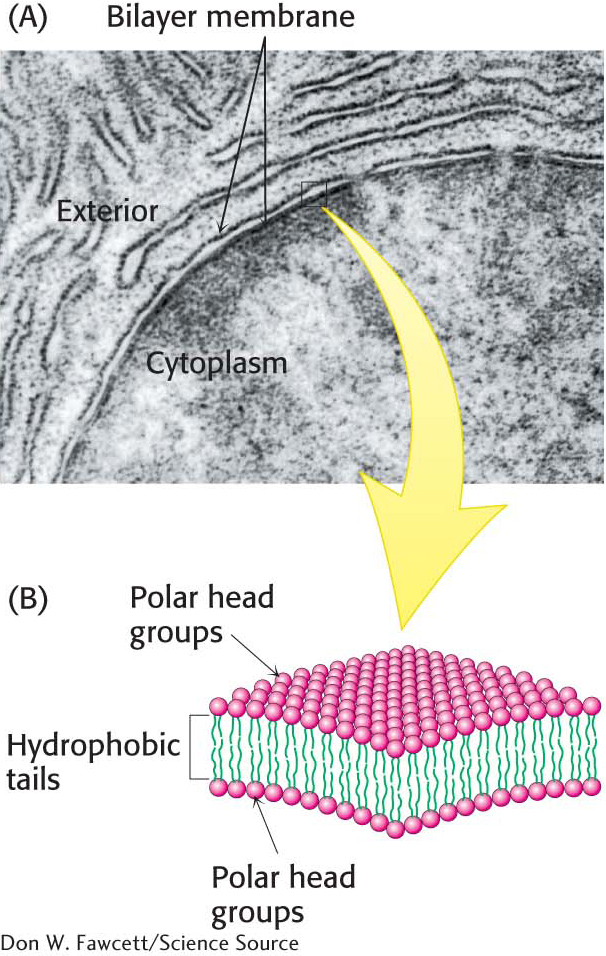
Recall from our earlier examination of membrane lipids that, although the repertoire of lipids is extensive, these lipids possess common structural elements: they are amphipathic molecules with a hydrophilic (polar) head group and a hydrophobic hydrocarbon tail. Membrane formation is a consequence of the amphipathic nature of the molecules. Their polar head groups favor contact with water, whereas their hydrocarbon tails interact with one another in preference to water. How can molecules with these preferences arrange themselves in aqueous solutions? The favored structure for most phospholipids and glycolipids in aqueous media is a lipid bilayer, composed of two lipid sheets. The hydrophobic tails of each individual sheet interact with one another, forming a hydrophobic interior that acts as a permeability barrier. The hydrophilic head groups interact with the aqueous medium on each side of the bilayer. The two opposing sheets are called leaflets (Figure 12.1).
Lipid bilayers form spontaneously by a self-
 CLINICAL INSIGHT
CLINICAL INSIGHTLipid Vesicles Can Be Formed from Phospholipids
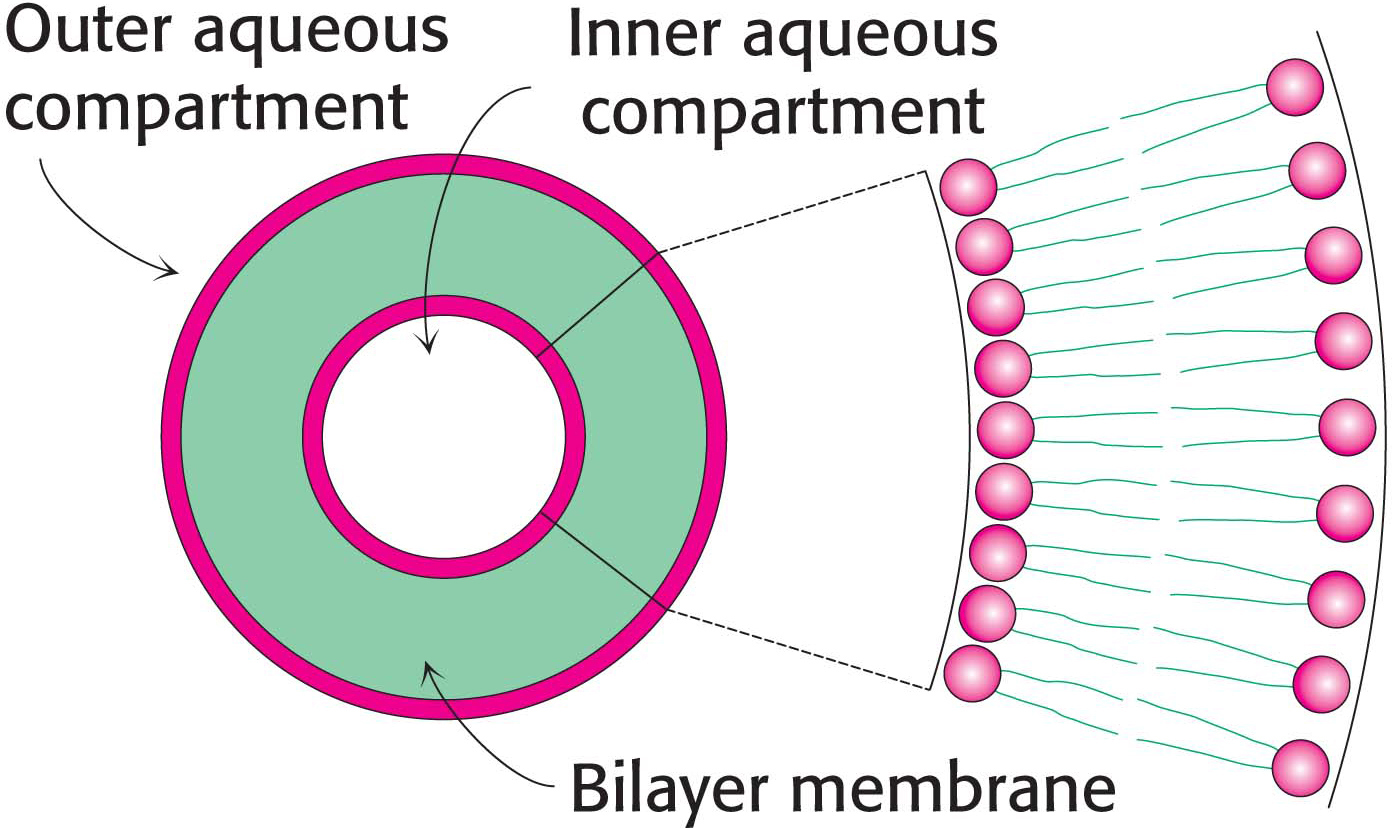
The propensity of phospholipids to form membranes has been used to create an important experimental and clinical tool. Lipid vesicles, or liposomes, are aqueous compartments enclosed by a lipid bilayer (Figure 12.2). These structures can be used to study membrane permeability or to deliver chemicals to cells. Liposomes are formed by suspending a membrane lipid in an aqueous medium and then sonicating (i.e., agitating by high-
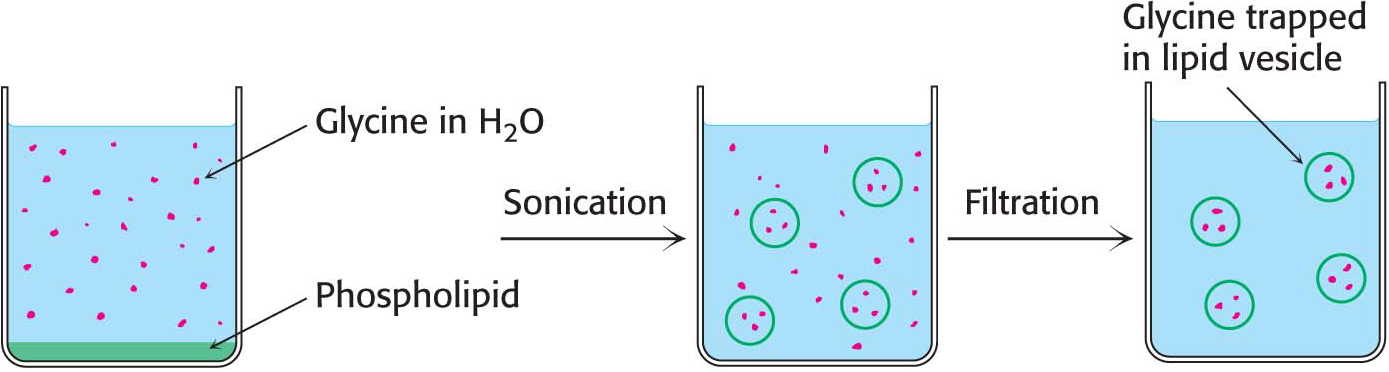
Experiments are underway to develop clinical uses for liposomes. DNA-
Lipid Bilayers Are Highly Impermeable to Ions and Most Polar Molecules
✓ 2 Explain why membranes are impermeable to most substances.
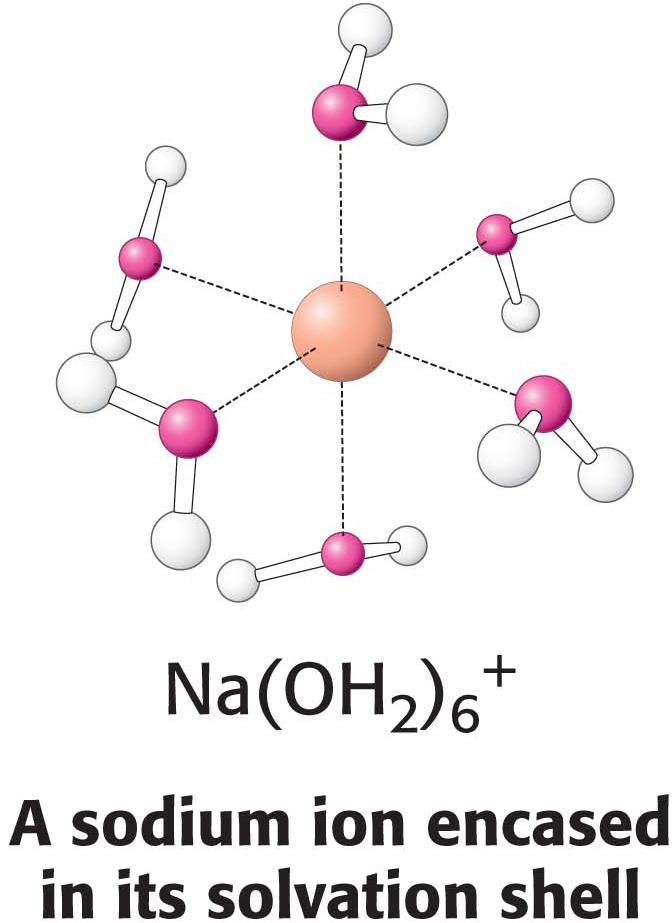
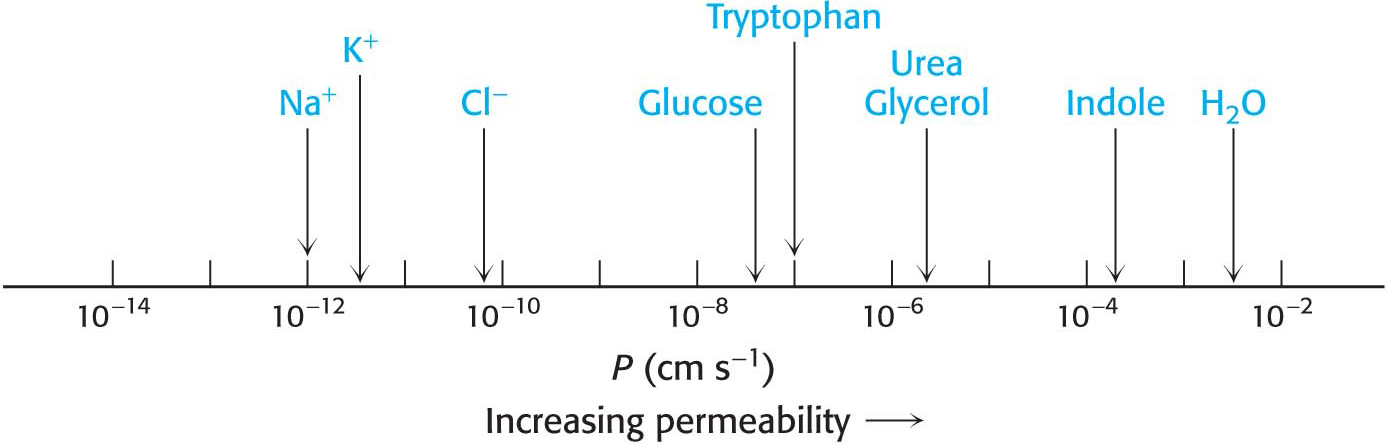
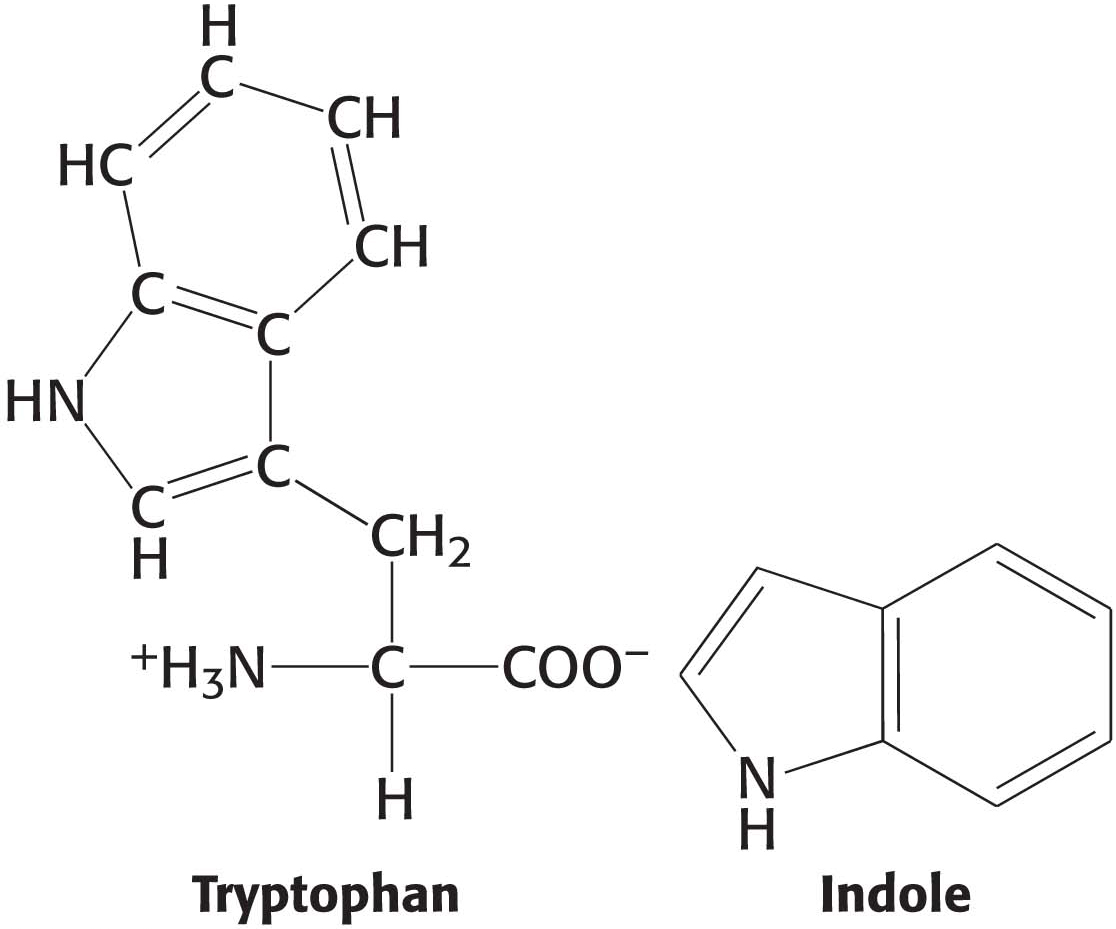
The results of permeability studies of lipid bilayers have shown that lipid bilayer membranes have a very low permeability for ions and most polar molecules. The ability of molecules to move through a lipid environment, such as a membrane, is quite varied (Figure 12.4). For example, Na+ and K+ traverse these membranes 109 times more slowly than H2O. Tryptophan, a zwitterion at pH 7, crosses the membrane 103 times more slowly than indole, a structurally related molecule that lacks ionic groups. In fact, experiments show that the permeability of small molecules is correlated with their relative solubilities in water and nonpolar solvents. This relation suggests that a small molecule might traverse a lipid bilayer membrane in the following way: first, it sheds the water with which it is associated, called the solvation shell; then, it dissolves in the hydrocarbon core of the membrane; and, finally, it diffuses through this core to the other side of the membrane, where it is resolvated by water. An ion such as Na+ cannot cross the membrane, because the replacement of its shell of polar water molecules by nonpolar interactions with the membrane interior is highly unfavorable energetically.