12.4 Lipids and Many Membrane Proteins Diffuse Laterally in the Membrane
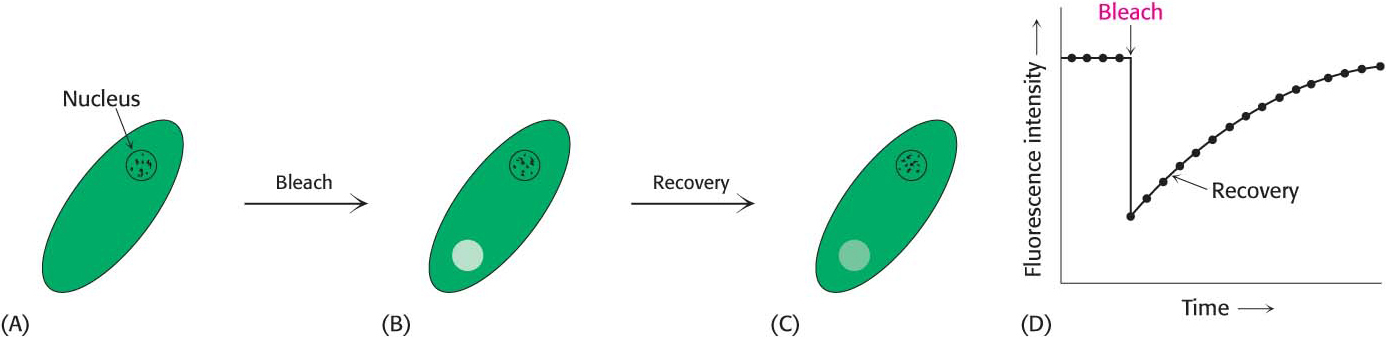
Membranes are not rigid, static structures. On the contrary, lipids and many membrane proteins are constantly in motion, a process called lateral diffusion. The rapid lateral movement of membrane constituents has been visualized with the use of fluorescence microscopy and the technique of fluorescence recovery after photobleaching (FRAP; Figure 12.14). First, a cell-surface component, such as a protein or the head group of a phospholipid, is fluorescently labeled. A small region of the cell surface (~3 μm2) is viewed through a fluorescence microscope. The fluorescent molecules in this region are then destroyed (bleached) by an intense pulse of light from a laser. The fluorescence of this region is subsequently monitored as a function of time. If the labeled component is mobile, bleached molecules leave and unbleached molecules enter the affected region, which results in an increase in the fluorescence intensity. The rate of recovery of fluorescence depends on the lateral mobility of the fluorescence-labeled component. Such experiments show that a phospholipid molecule moves an average distance of 2 μm in 1 s. This rate means that a lipid molecule can travel from one end of a bacterium to the other in a second. In contrast, proteins vary markedly in their lateral mobility. Some proteins are nearly as mobile as lipids, whereas others are virtually immobile. Proteins are immobilized by attachment to the cytoskeleton or structural components outside the cell, called the extracellular matrix.
Page 212
On the basis of the mobility of proteins in membranes, in 1972 S. Jonathan Singer and Garth Nicolson proposed a fluid mosaic model to describe the overall organization of biological membranes. The essence of their model is that membranes are two-dimensional solutions of oriented lipids and globular proteins. The lipid bilayer has a dual role: it is both a solvent for integral membrane proteins and a permeability barrier. Membrane proteins are free to diffuse laterally in the lipid matrix unless restricted by special interactions.
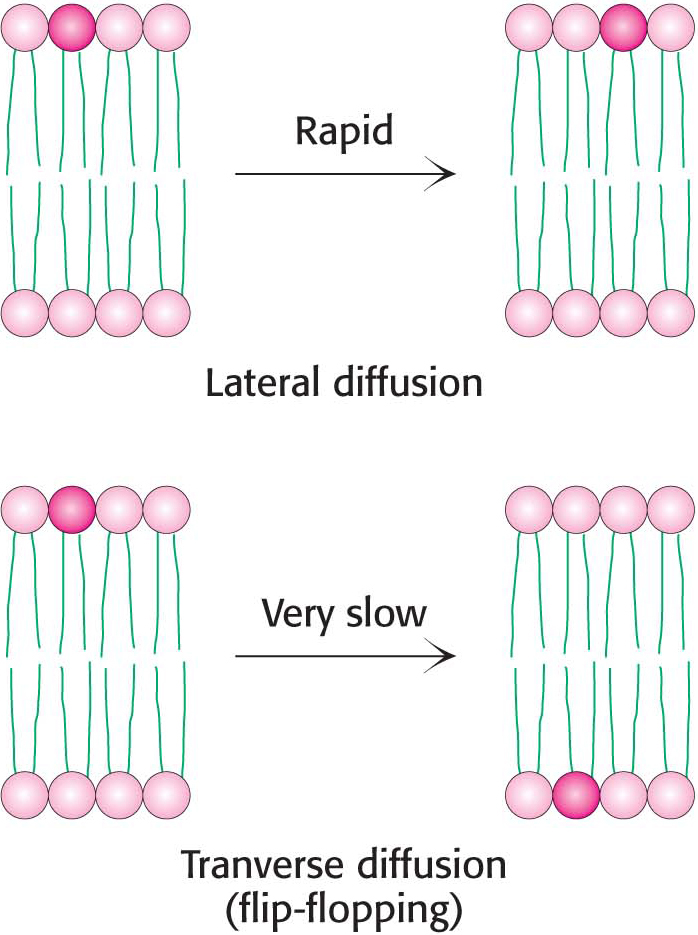
Although the lateral diffusion of membrane components can be rapid, the spontaneous rotation of lipids from one face of a membrane to the other is a very slow process. The transition of a molecule from one membrane surface to the other is called transverse diffusion or flip-flopping. The results of experiments show that a phospholipid molecule flip-flops once in several hours. Thus, a phospholipid molecule takes about 109 times as long to flip-flop across a membrane as it takes to diffuse a distance of 50 Å in the lateral direction (Figure 12.15). The free-energy barriers to flip-flopping are even larger for protein molecules than for lipids because proteins have more extensive polar regions. In fact, the flip-flopping of a protein molecule has not been observed. Hence, membrane asymmetry can be preserved for long periods.