Receptor Dimerization May Result in Tyrosine Kinase Recruitment
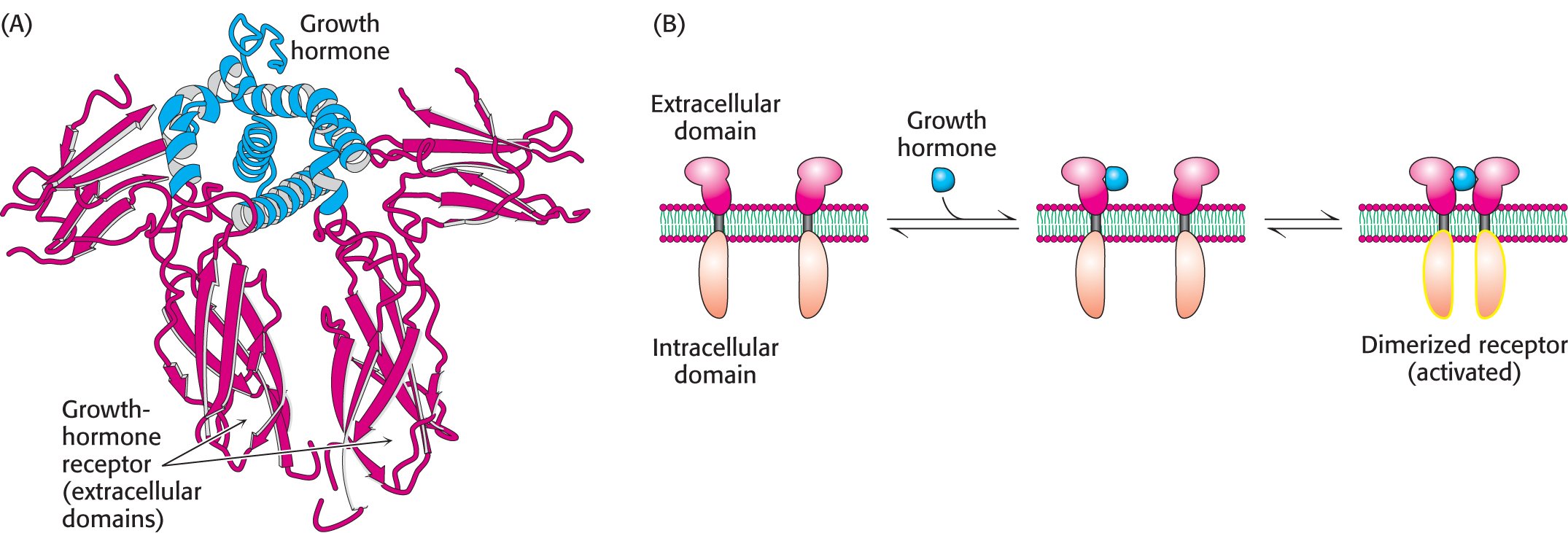
The growth-hormone receptor is a well-studied example of a receptor that dimerizes on ligand binding and subsequently recruits tyrosine kinases to propagate the signal. Growth hormone is a monomeric protein of 217 amino acids that is required for cell division and growth. Human growth hormone (HGH) is abused by athletes who believe that HGH treatments will enhance their performance, although there is little evidence to support such beliefs. The growth-hormone receptor has an extracellular domain, a single membrane-spanning helix, and an intracellular domain. In the absence of bound hormone, the receptor is present as a monomer. Each hormone molecule binds to the extracellular domains of two receptor molecules, thus promoting the formation of a dimer of the receptor (Figure 13.13).
DID YOU KNOW?
Overstimulation of the growth-hormone signal-transduction pathway can lead to pathological conditions. Acromegaly is a rare hormonal disorder resulting from the overproduction of growth hormone in middle age. A common characteristic of acromegaly is enlargement of the face, hands, and feet. Excessive production of growth hormone in children results in gigantism. André the Giant, who played the beloved giant Fezzik in rob reiner’s classic film The Princess Bride suffered from gigantism.
Page 234
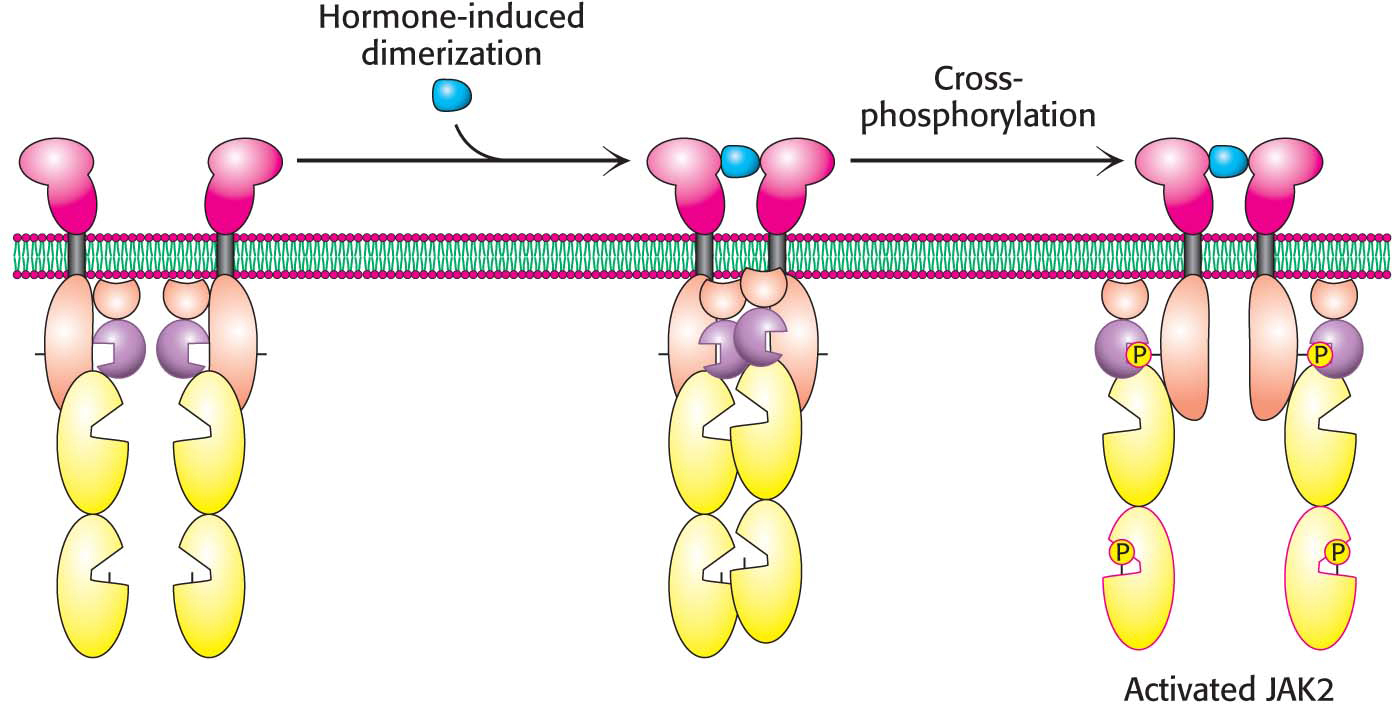
Dimerization of the extracellular domains of the receptor brings together the intracellular domains as well. Associated with each intracellular domain is a molecule of a tyrosine protein kinase, termed Janus kinase 2 (JAK2), in an unactivated form (Figure 13.14). Tyrosine kinases phosphorylate proteins on the hydroxyl group of tyrosine residues. Dimerization of the growth-hormone receptors brings together the JAK2 proteins associated with each intracellular domain. Each of the kinases phosphorylates its partner, resulting in the activation of the kinases.
When activated by cross-phosphorylation, JAK2 can phosphorylate other substrates, such as a regulator of gene expression called STAT5 (STAT for signal transducers and activators of transcription). Phosphorylated STAT5 moves to the nucleus, where it binds to the DNA-binding sites to regulate gene expression. A signal received on the outside of the cell membrane is forwarded to the nucleus for action.
Page 235
 CLINICAL INSIGHT
CLINICAL INSIGHTSome Receptors Contain Tyrosine Kinase Domains Within Their Covalent Structures
Some growth factors and hormones—such as epidermal growth factor (EGF), platelet-derived growth factor, and insulin—bind to the extracellular domains of transmembrane receptors that have tyrosine kinase domains within their intracellular domains. These receptor tyrosine kinases (RTKs) signal by mechanisms quite similar to those described for the pathway initiated by the growth-hormone receptor discussed in the preceding subsection. Humans have 58 known genes encoding receptor tyrosine kinases. Mutations in these receptors cause a range of pathologies, including arteriosclerosis, cancer, inflammation, and type 2 diabetes.
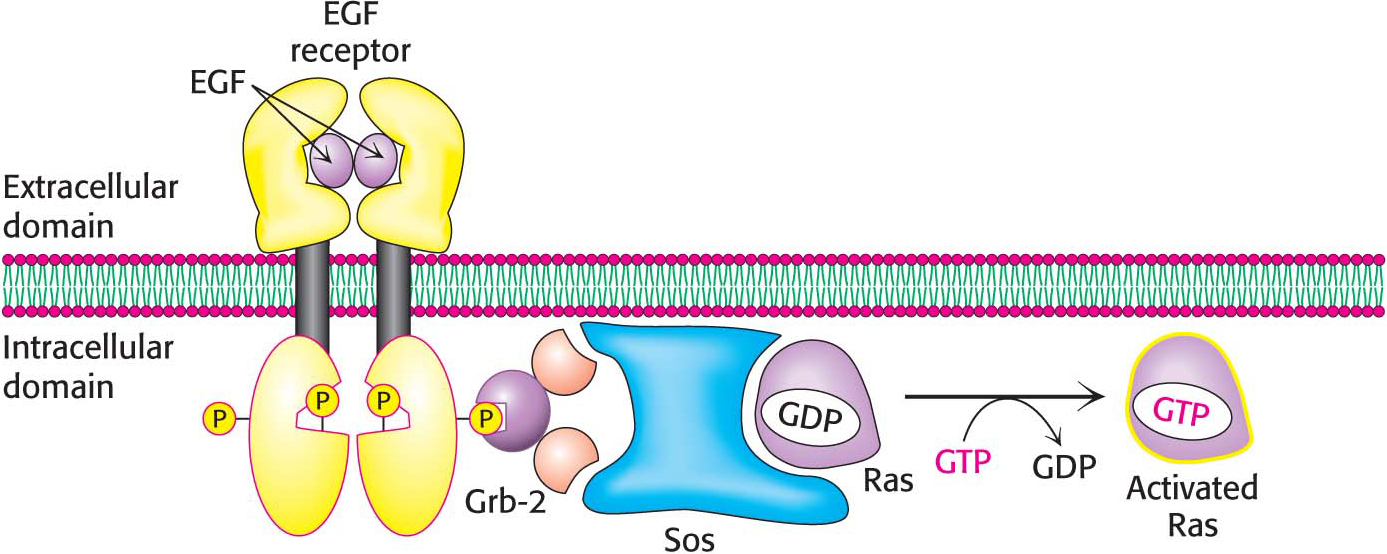
Consider, for example, epidermal growth factor, a 6-kDa polypeptide that stimulates the growth of epidermal and epithelial cells by binding to the epidermal-growth-factor receptor, a single polypeptide chain consisting of an extracellular growth hormone binding domain, a helix that spans the membrane, and an intracellular kinase domain. The receptor is monomeric and enzymatically inactive in the absence of the growth factor. The binding of EGF to its extracellular domain causes the receptor to dimerize and undergo cross-phosphorylation and activation.
How is the signal transferred beyond the receptor tyrosine kinase? A key adaptor protein, called Grb-2, links the phosphorylation of the EGF receptor to the stimulation of cell growth through a chain of protein phosphorylations (Figure 13.15). On phosphorylation of the receptor, Grb-2 binds to the phosphotyrosine residues of the receptor tyrosine kinase. Grb-2 then recruits a protein called Sos. Sos, in turn, binds to Ras and activates it. Ras is a very prominent signal-transduction component that we will consider shortly. Finally, Ras, in its activated form, binds to other components of the molecular circuitry, leading to the activation of the specific protein kinases that phosphorylate specific targets that promote cell growth. We see here another example of how a signal-transduction pathway is constructed. Specific protein–protein interactions link the original ligand-binding event to the final result—the stimulation of cell growth.
Page 236
Ras Belongs to Another Class of Signaling G Proteins
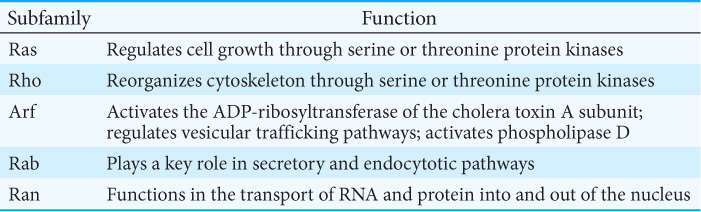
The signal-transduction protein Ras is a member of a family of signal proteins—the small G proteins, or small GTPases. This large superfamily of proteins—grouped into subfamilies called Ras, Rho, Arf, Rab, and Ran—plays a major role in a host of cell functions, including growth, differentiation, cell motility, cytokinesis, and the transport of materials throughout the cell (Table 13.3). Like their relatives the heterotrimeric G proteins, the small G proteins cycle between an active GTP-bound form and an inactive GDP-bound form. They differ from the heterotrimeric G proteins in being smaller (20–25 kDa compared with 30–35 kDa) and monomeric.
Recall that Sos is the immediate upstream link to Ras in the circuit conveying the EGF signal (Figure 13.15). How does Sos activate Ras? Sos binds to Ras, reaches into the nucleotide-binding pocket, and opens it up, allowing GDP to escape and GTP to enter in its place. Sos is referred to as a guanine-nucleotide exchange factor (GEF).
Like the Gα protein, Ras possesses an intrinsic GTPase activity, which serves to terminate the signal and return the system to the inactive state. This activity is slow but is augmented by helper proteins termed GTPase-activating proteins (GAPs). The guanine-nucleotide exchange factors and the GTPase-activating proteins allow the G-protein cycle to proceed with rates appropriate for a balanced level of downstream signaling.


 CLINICAL INSIGHT
CLINICAL INSIGHT