
13.4 Metabolism in Context: Insulin Signaling Regulates Metabolism
Insulin is among the principal hormones that regulate metabolism, and we will examine the effects of this hormone on metabolic pathways many times in the course of our study of biochemistry. This section presents an overview of its signal-
The Insulin Receptor Is a Dimer That Closes Around a Bound Insulin Molecule
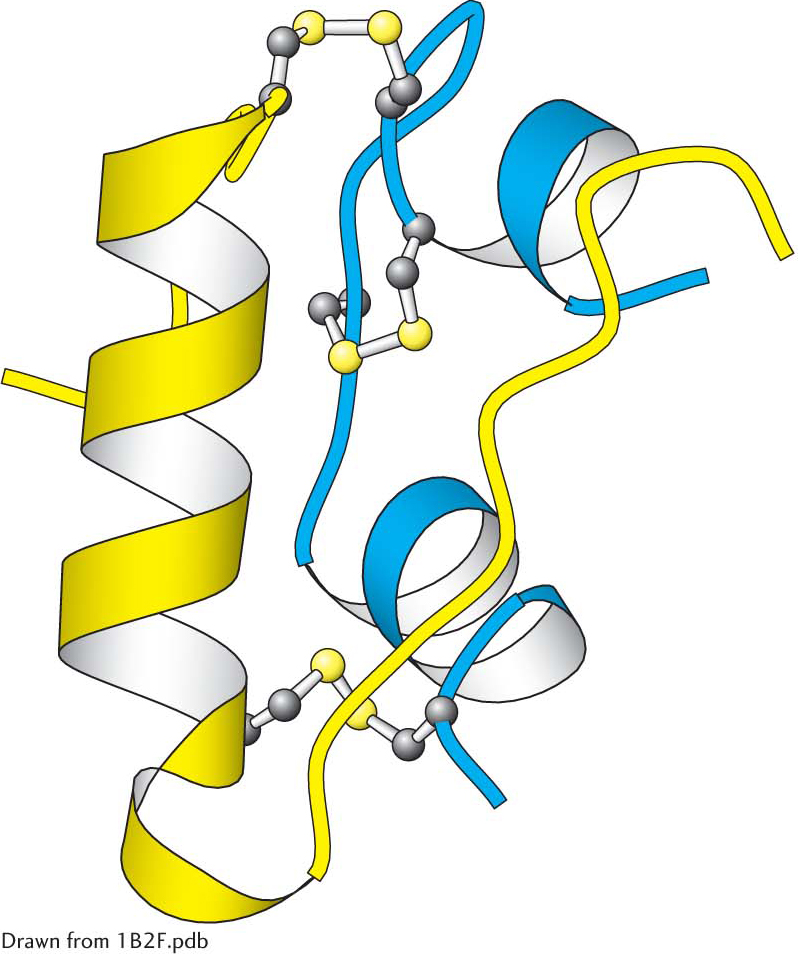
Insulin is a peptide hormone that consists of two chains, linked by two disulfide bonds (Figure 13.16). The insulin receptor is a member of the receptor tyrosine kinase class of membrane proteins. Unlike the other members of the receptor tyrosine kinase class, however, the insulin receptor exists as a dimer even in the absence of insulin. Each subunit consists of one α chain and one β chain linked to one another by a single disulfide bond. Each α subunit lies completely outside the cell, whereas each β subunit starts in the extracellular domain and spans the membrane with an α helix to the cytoplasmic side, where the kinase domain resides. The two α subunits are linked to one another with disulfide bonds (Figure 13.17).
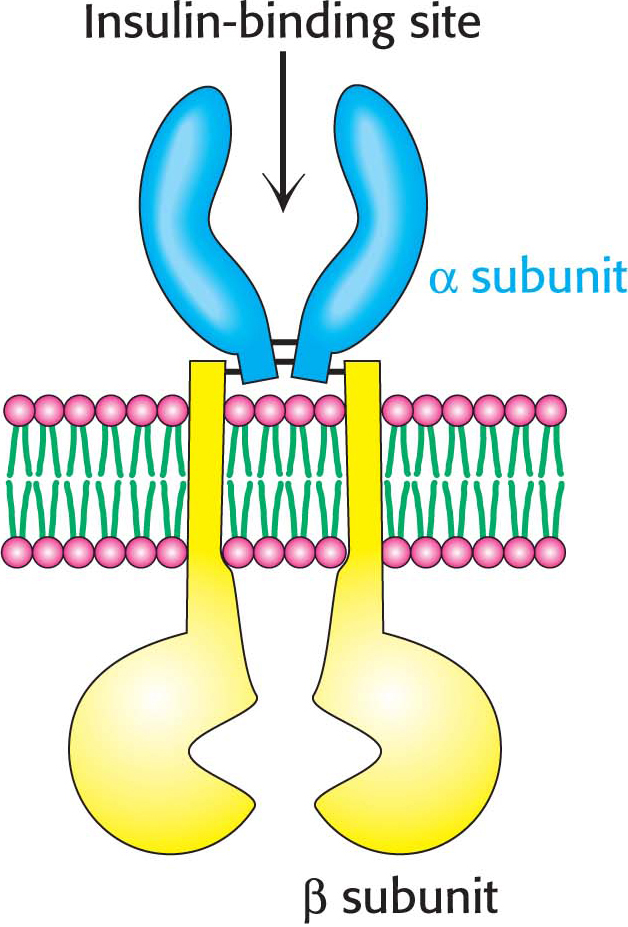
The Activated Insulin-Receptor Kinase Initiates a Kinase Cascade
Insulin binding causes a change in quaternary structure that results in cross-
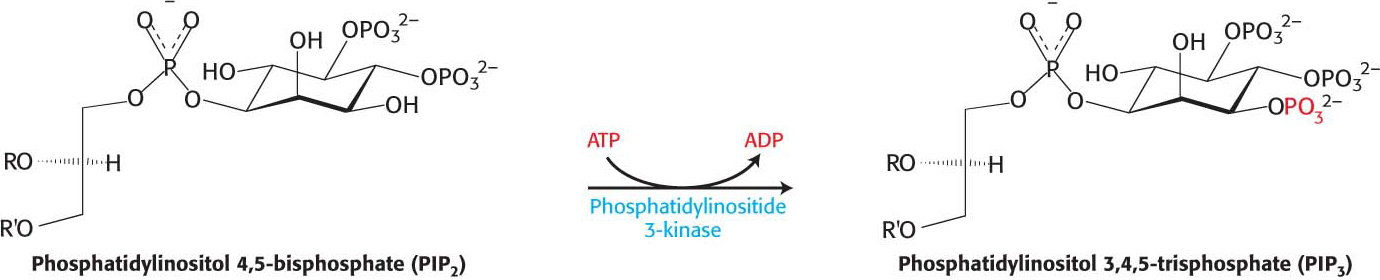
In their phosphorylated form, the IRS molecules act as adaptor proteins (Figure 13.18). The phosphotyrosine residues in the IRS proteins are recognized by other proteins, including a lipid kinase that phosphorylates phosphatidylinositol 4,5-
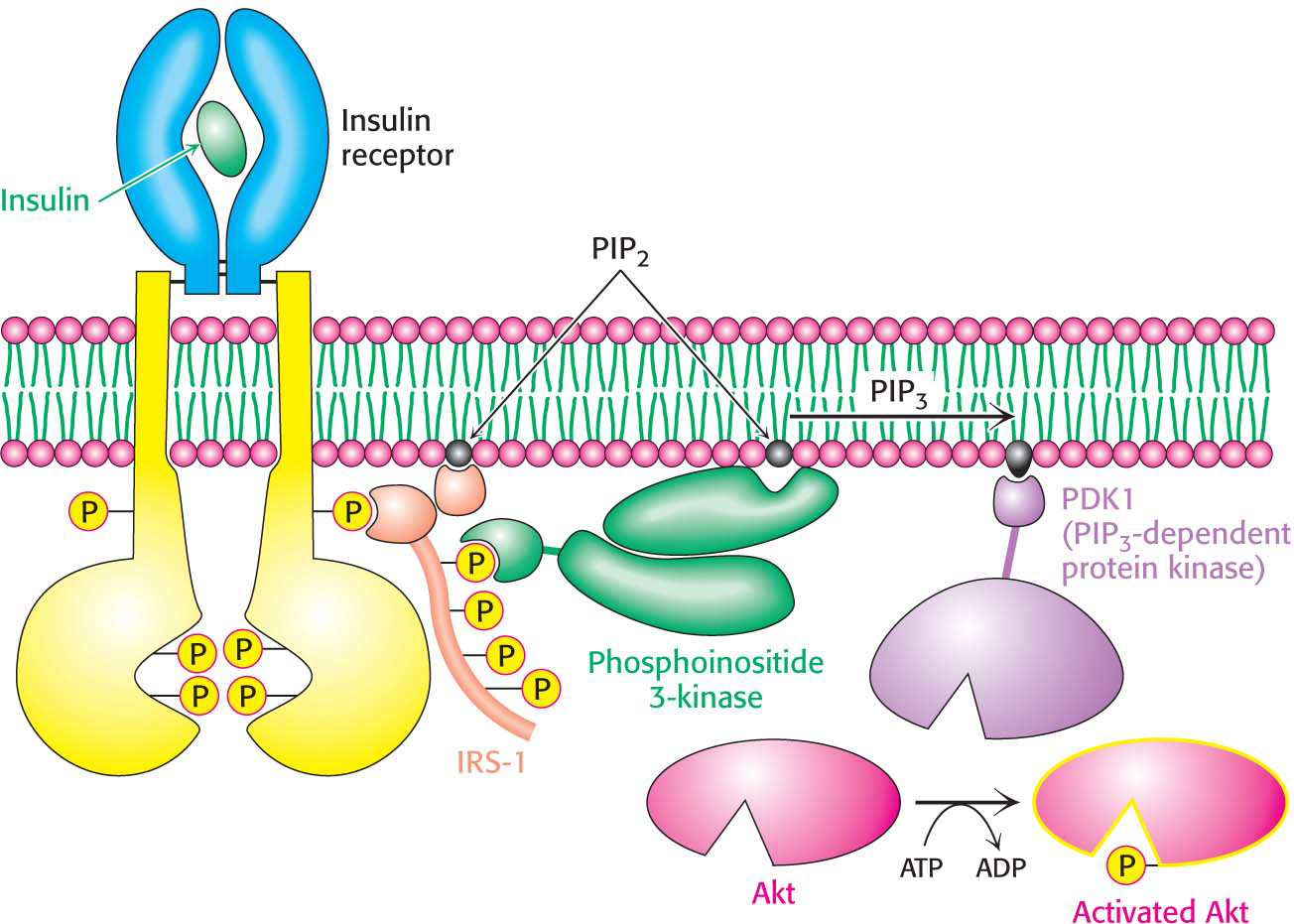
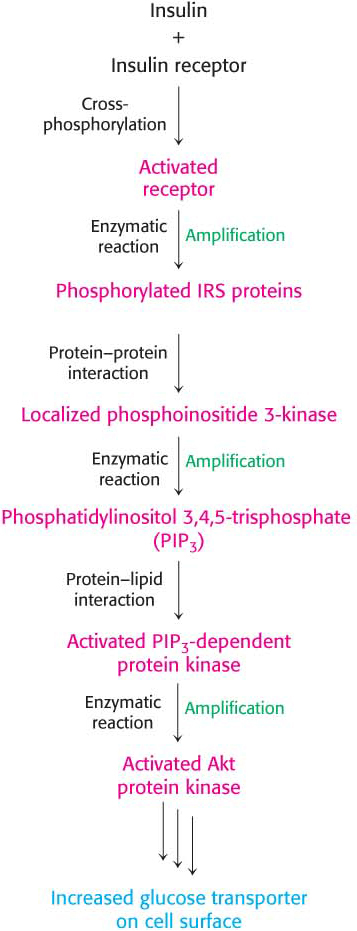
The cascade initiated by the binding of insulin to the insulin receptor is summarized in Figure 13.20. The signal is amplified at several stages along this pathway. Because the activated insulin receptor itself is a protein kinase, each activated receptor can phosphorylate multiple IRS molecules. Activated enzymes further amplify the signal in at least two of the subsequent steps. Thus, a small increase in the concentration of circulating insulin can produce a robust intracellular response. Note that, as complicated as the pathway described here is, it is substantially less elaborate than the full network of pathways initiated by insulin.
Insulin Signaling Is Terminated by the Action of Phosphatases
QUICK QUIZ 2
Why does it make good physiological sense for insulin to increase the number of glucose transporters in the cell membrane?
Insulin signifies the fed state. Its presence leads to the removal of glucose from the blood for storage or metabolism. Increasing the number of glucose transporters available makes these biochemical processes more efficient.
We have seen that the activated G protein promotes its own inactivation by the release of a phosphoryl group from GTP. In contrast, proteins phosphorylated on serine, threonine, or tyrosine residues, such as insulin-
