13.5 Calcium Ion Is a Ubiquitous Cytoplasmic Messenger
We have already seen that Ca2+ is a component of one signal-transduction circuit, the phosphoinositide cascade. Indeed, Ca2+ is itself an intracellular messenger in many eukaryotic signal-transducing pathways.
Calmodulin (CaM), a 17-kDa protein with four calcium-binding sites, serves as a calcium sensor in nearly all eukaryotic cells. Calmodulin is activated by the binding of Ca2+ when the cytoplasmic calcium level is raised. Calmodulin is a member of the EF-hand protein family. The EF hand is a Ca2+-binding motif that consists of a helix, a loop, and a second helix. This motif, originally discovered in the protein parvalbumin, was named the EF hand because the helices designated E and F in parvalbumin that form the calcium-binding motif are positioned like the forefinger and thumb of the right hand (Figure 13.21).
Page 239
The Ca2+–calmodulin complex stimulates a wide array of enzymes, pumps, and other target proteins. Two targets are especially noteworthy: one that propagates the Ca2+ signal and another that abrogates it. The binding of Ca2+–calmodulin to a calmodulin-dependent protein kinase (CaM kinase) activates the kinase and enables it to phosphorylate a wide variety of target proteins. CaM kinases regulate the metabolism of fuel, ionic permeability, neurotransmitter synthesis, and neurotransmitter release through the action of the Ca2+–calmodulin complex. The plasma-membrane Ca2+–ATPase pump is another important target of Ca2+–calmodulin. Stimulation of the pump by Ca2+–calmodulin drives the calcium level down to restore a low-calcium basal state to the cell, thus helping to terminate the signal.

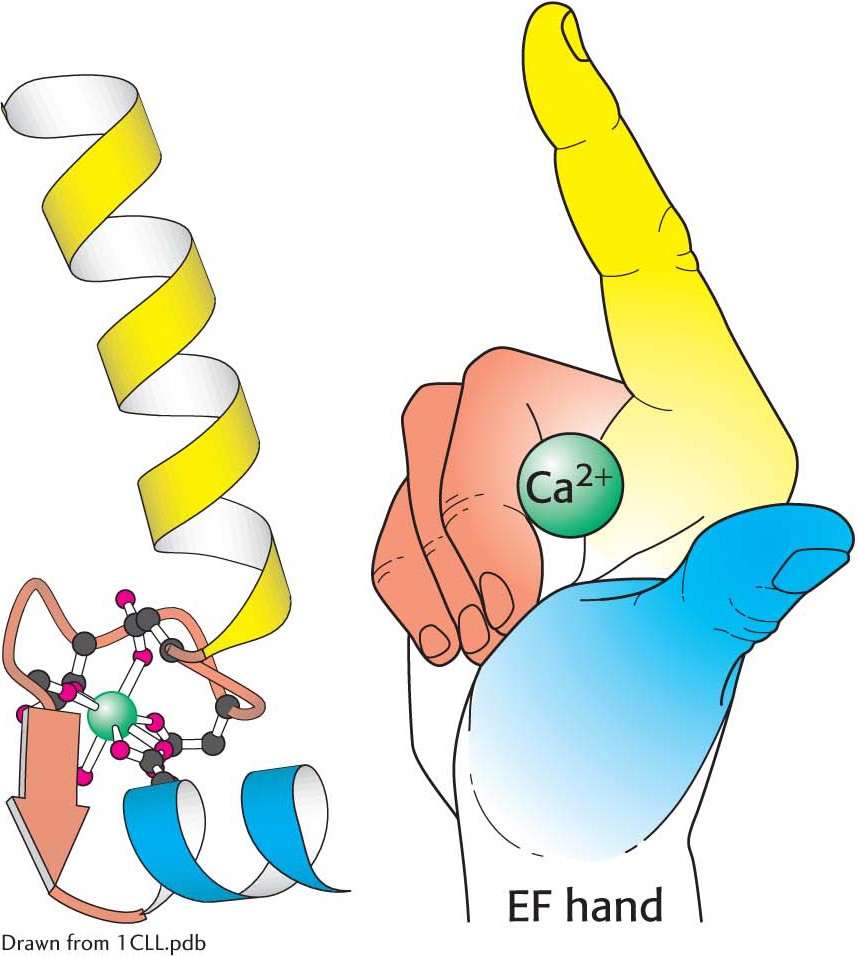
 An EF hand. Formed by a helix-
An EF hand. Formed by a helix-