
15.2 Metabolism Is Composed of Many Interconnecting Reactions

Metabolism is a linked series of chemical reactions that begins with a particular biomolecule and converts it into some other required biomolecule in a carefully defined fashion (Figure 15.2). These metabolic pathways process a biomolecule from a starting point (glucose, for instance) to an end point (carbon dioxide, water, and biochemically useful energy, in regard to glucose) without the generation of wasteful or harmful side products. There are many such defined pathways in the cell (Figure 15.3), together called intermediary metabolism, and we will examine many of them in some detail later. These pathways are interdependent—

Metabolism Consists of Energy-Yielding Reactions and Energy-Requiring Reactions
We can divide metabolic pathways into two broad classes: (1) those that convert energy from fuels into biologically useful forms, such as ATP or ion gradients, and (2) those that require inputs of energy to proceed. Although this division is often imprecise, it is nonetheless a useful distinction in an examination of metabolism. Those reactions that transform fuels into cellular energy are called catabolic reactions or, more generally, catabolism:

Those reactions that require energy—

Some pathways can be either anabolic or catabolic, depending on the energy conditions in the cell. They are referred to as amphibolic pathways.
An important general principle of metabolism is that, although biosynthetic and degradative pathways often have reactions in common, the regulated, irreversible reactions of each pathway are almost always distinct from each other. This separation is necessary for energetic reasons, as will be evident in subsequent chapters. It also facilitates the control of metabolism.
A Thermodynamically Unfavorable Reaction Can Be Driven by a Favorable Reaction
How are specific pathways constructed from individual reactions? A pathway must satisfy minimally two criteria: (1) the individual reactions must be specific, and (2) the entire set of reactions that constitute the pathway must be thermodynamically favored. A reaction that is specific will yield only one particular product or set of products from its reactants. For example, glucose can undergo step-
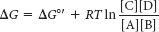
Thus, the ΔG of a reaction depends on the nature of the reactants and products (expressed by the ΔG°′ term, the standard free-
An important thermodynamic fact is that the overall free-

Under standard conditions, A cannot be spontaneously converted into B and C, because ΔG°′ is positive. However, the conversion of B into D under standard conditions is thermodynamically feasible. Because free-