
15.3 ATP Is the Universal Currency of Free Energy
✓ 3 Identify the factors that make ATP an energy-
✓ 4 Explain how ATP can power reactions that would otherwise not take place.
Just as commerce is facilitated by the use of a common monetary currency, the commerce of the cell—
ATP Hydrolysis Is Exergonic
ATP is a nucleotide consisting of adenine, a ribose, and a triphosphate unit (Figure 15.4). In considering the role of ATP as an energy carrier, we can focus on its triphosphate moiety. ATP is an energy-

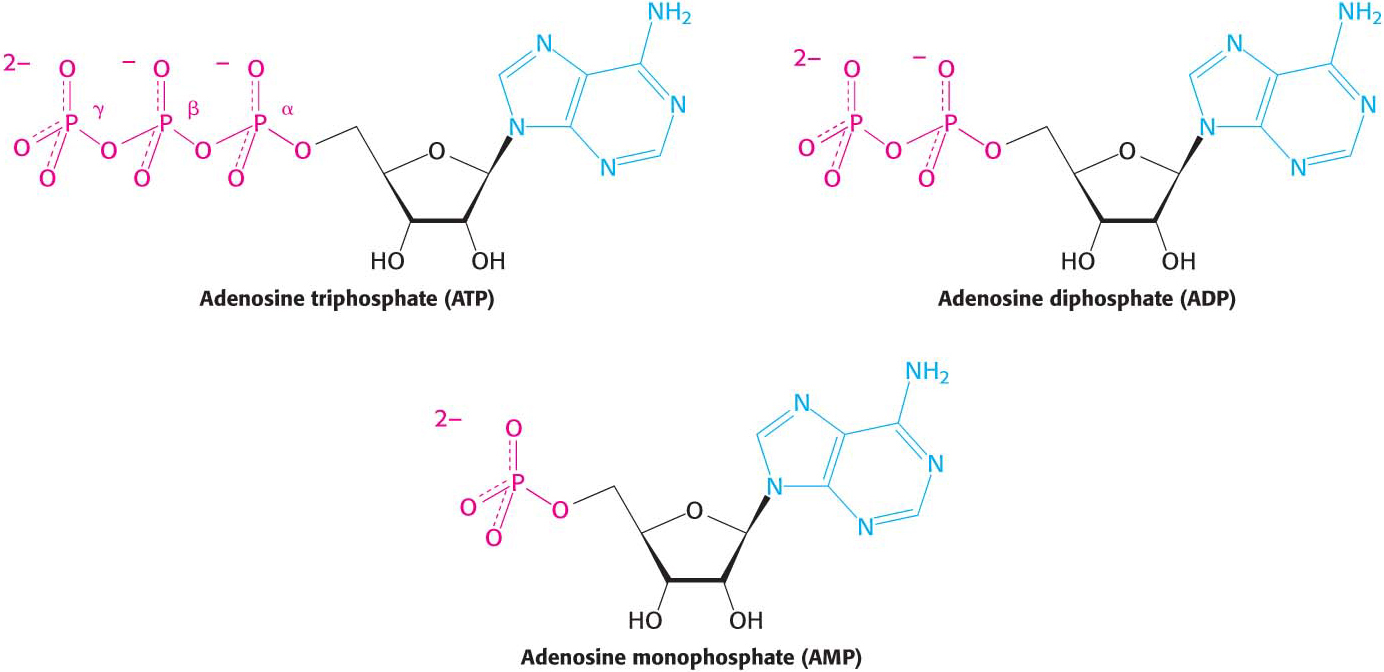
The precise ΔG°′ for these reactions depends on the ionic strength of the medium and on the concentrations of Mg2+ and other metal ions in the medium (problems 27 and 32). Under typical cellular concentrations, the actual ΔG for these hydrolyses is approximately −50 kJ mol−1 (−12 kcal mol−1).
The free energy liberated in the hydrolysis of ATP is harnessed to drive reactions that require an input of free energy, such as muscle contraction. In turn, ATP is formed from ADP and Pi when fuel molecules are oxidized in chemotrophs or when light is trapped by phototrophs. This ATP–
ATP Hydrolysis Drives Metabolism by Shifting the Equilibrium of Coupled Reactions
An otherwise unfavorable reaction can be made possible by coupling to ATP hydrolysis. Consider an endergonic chemical reaction, a reaction that would not occur without an input of free energy, but yet is required for biosynthetic pathway.
Suppose that the standard free energy of the conversion of compound A into compound B is + 16.7 kJ mol−1 (+4.0 kcal mol−1):
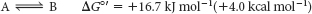
The equilibrium constant K′eq of this reaction at 25°C is related to ΔG°′ (in units of kilojoules per mole) by
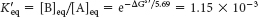
Thus, the net conversion of A into B cannot take place when the molar ratio of B to A is equal to or greater than 1.15 × 10−3. However, A can be converted into B under these conditions if the reaction is coupled to the hydrolysis of ATP. Under standard conditions, the ΔG°′ of hydrolysis is approximately −30.5 kJ mol−1 (−7.3 kcal mol−1). The new overall reaction is

Its free-
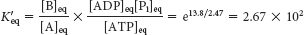
At equilibrium, the ratio of [B] to [A] is given by
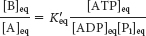
which means that the hydrolysis of ATP enables compound A to be converted into compound B until the [B]/[A] ratio reaches a value of 2.67 × 102. This equilibrium ratio is strikingly different from the value of 1.15 × 10−3 for the reaction A → B in the absence of ATP hydrolysis. In other words, coupling the hydrolysis of ATP with the conversion of A into B under standard conditions has changed the equilibrium ratio of B to A by a factor of about 105. If we were to use the ΔG° of hydrolysis of ATP under cellular conditions [−50.2 kJ mol−1 (−12 kcal mol−1)] in our calculations instead of ΔG°′, the change in the equilibrium ratio would be even more dramatic, of the order of 108 (problem 31).
We see here the thermodynamic essence of ATP’s action as an energy-
Note that A and B in the preceding coupled reaction may be interpreted very generally, not only as different metabolites. For example, A and B may represent activated and unactivated conformations of a protein that is activated by phosphorylation with ATP. Through such changes in protein conformation, muscle proteins convert the chemical energy of ATP into the mechanical energy of muscle contraction. Alternatively, A and B may refer to the concentrations of an ion or molecule on the outside and inside of a cell, as in the active transport of a nutrient.
The High Phosphoryl-Transfer Potential of ATP Results from Structural Differences Between ATP and Its Hydrolysis Products
What makes ATP an efficient energy currency? To answer this question, we need a means of comparing the tendency of various organic compounds bearing a phosphoryl group to transfer the phosphoryl group to an acceptor molecule. A common means of comparison is to determine the amount of energy released when the phosphorylated compound transfers the phosphoryl group to water under standard conditions. The energy released is called the standard free energy of hydrolysis. Let us compare the standard free energy of hydrolysis of ATP with that of a phosphate ester, such as glycerol 3-
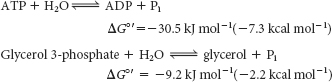
The magnitude of ΔG°′ for the hydrolysis of glycerol 3-
The high phosphoryl-
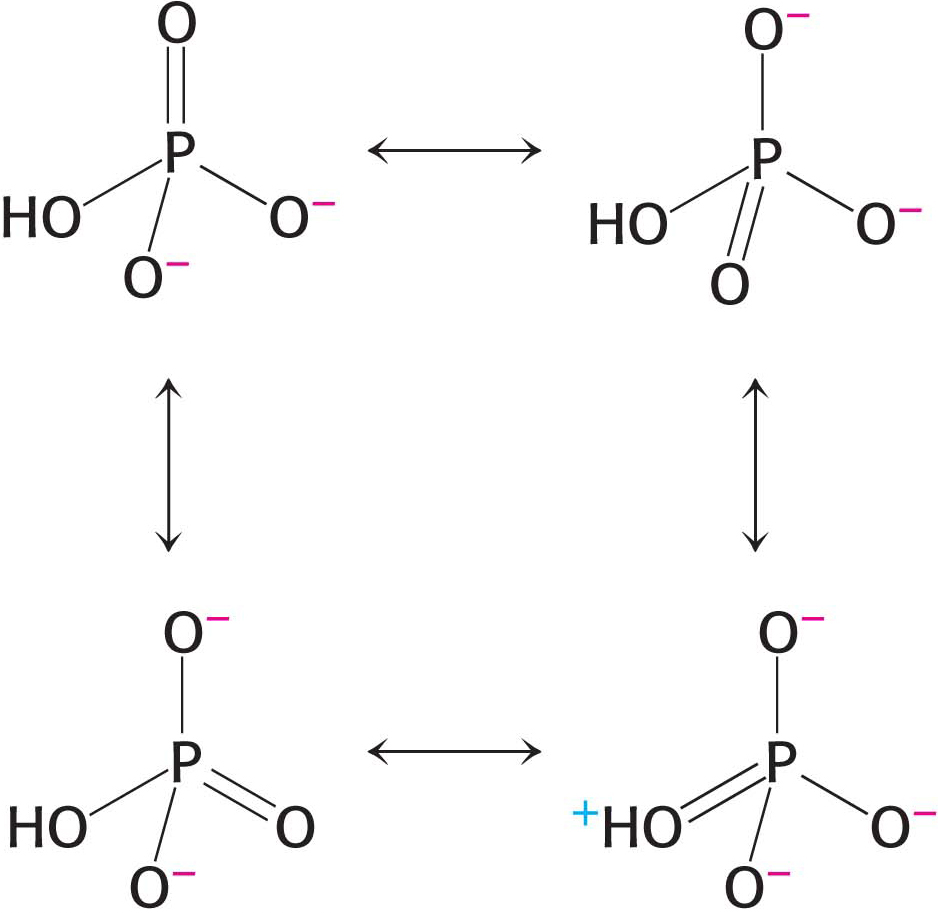 Figure 15.5 Resonance structures of orthophosphate.
Figure 15.5 Resonance structures of orthophosphate.Electrostatic Repulsion. At pH 7, the triphosphate unit of ATP carries about four negative charges (Figure 15.4). These charges repel one another because they are in close proximity. The repulsion between them is reduced when ATP is hydrolyzed.
Resonance Stabilization. Orthophosphate (Pi), one of the products of ATP hydrolysis, has greater resonance stabilization than do any of the phosphates in ATP. Orthophosphate has a number of resonance forms of similar energy (Figure 15.5), whereas the γ phosphoryl group of ATP has a smaller number (Figure 15.6). Forms like that shown on the right in Figure 15.5 are unfavorable because a positively charged oxygen atom is adjacent to a positively charged phosphorus atom, an electrostatically unfavorable juxtaposition.
 Figure 15.6 Improbable resonance structure. There are fewer resonance structures available to the γ-phosphate of ATP than to free orthophosphate.
Figure 15.6 Improbable resonance structure. There are fewer resonance structures available to the γ-phosphate of ATP than to free orthophosphate.Increase in Entropy. The entropy of the products is greater, in that there are now two molecules instead of a single ATP molecule. We disregard the molecule of water used to hydrolyze the ATP because given the high concentration (55.5 M), there is effectively no change in the concentration of water during the reaction.
Stabilization Due to Hydration. Water binds to ADP and Pi, stabilizing these molecules and thereby rendering the reverse reaction, the synthesis of ATP, less favorable.
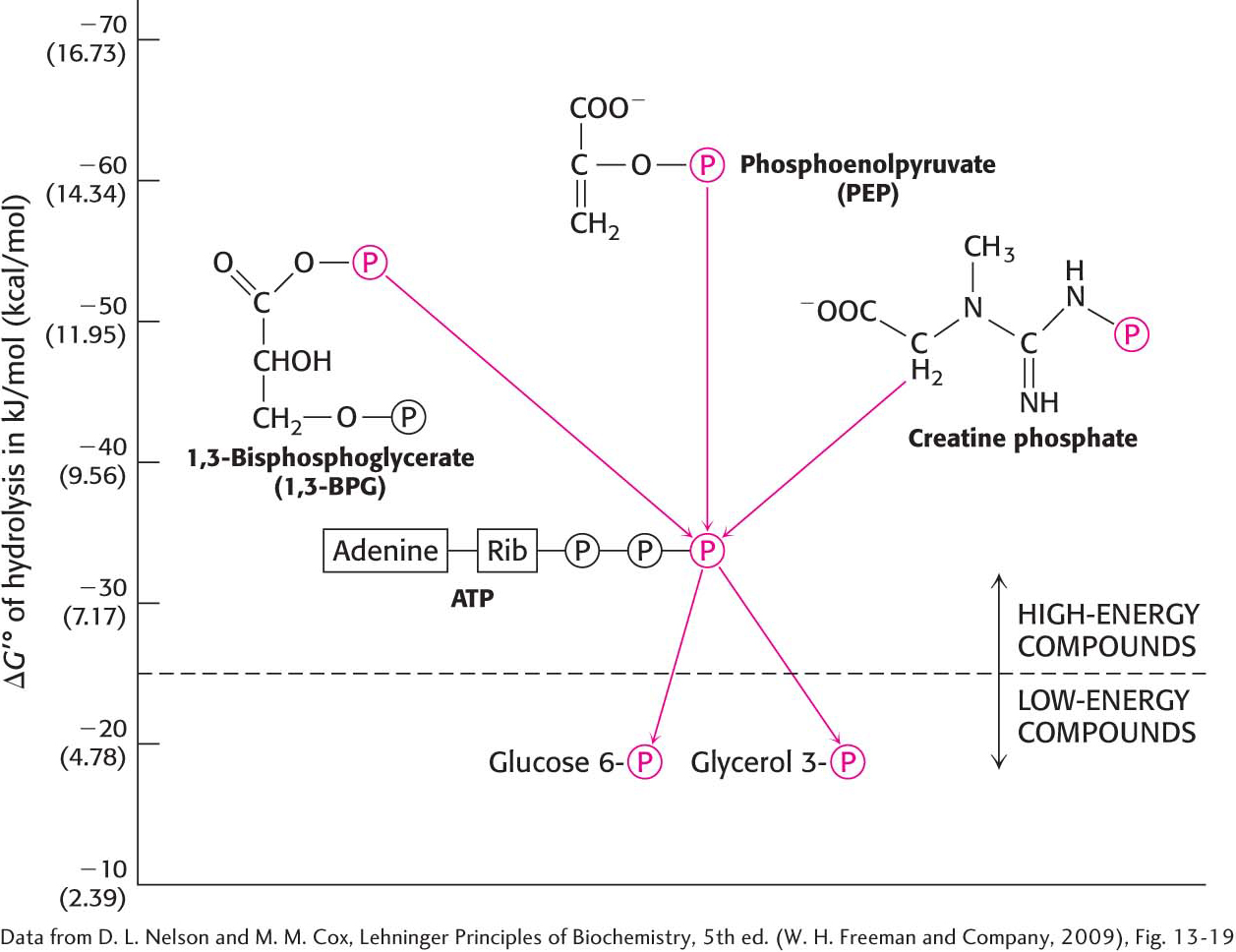
ATP is often called a high-
Phosphoryl-Transfer Potential Is an Important Form of Cellular Energy Transformation
QUICK QUIZ
What properties of ATP make it an especially effective phosphoryl-
Charge repulsion is reduced when a phosphoryl group is removed from ATP. The products of ATP hydrolysis have more resonance forms than does ATP. Entropy is increased when one molecule of ATP is converted into two product molecules, ADP and Pi. The products of ATP hydrolysis are more effectively stabilized by association with water than is ATP.
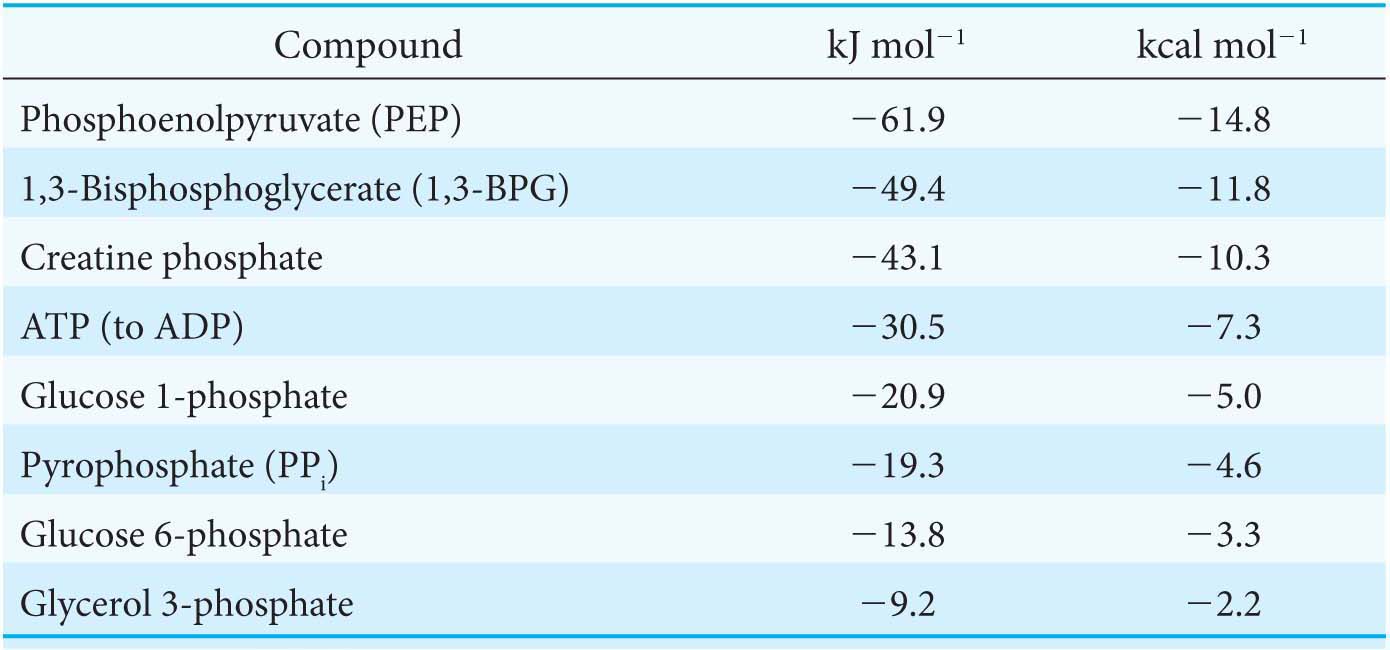
The standard free energies of hydrolysis provide a convenient means of comparing the phosphoryl-
 CLINICAL INSIGHT
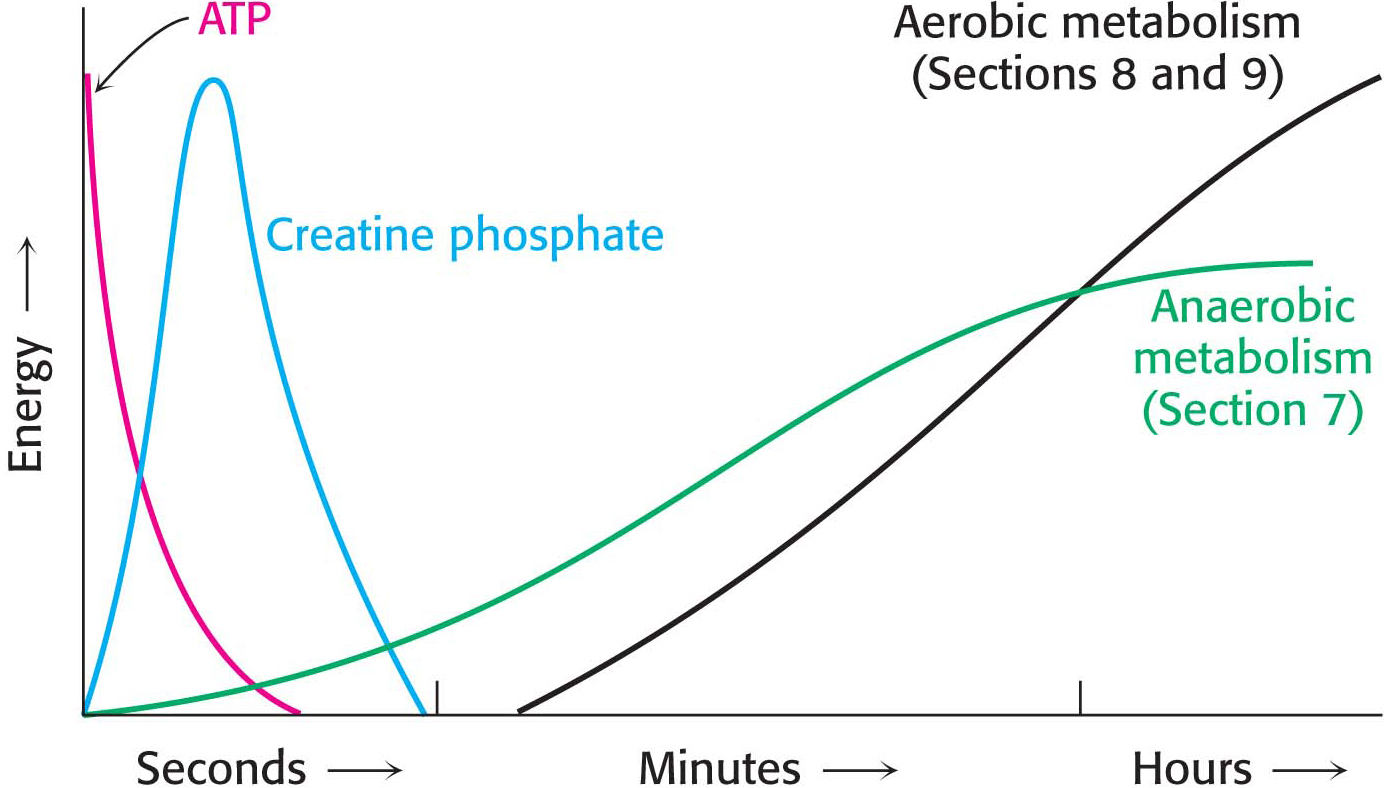
CLINICAL INSIGHTExercise Depends on Various Means of Generating ATP
At rest, muscle contains only enough ATP to sustain contractile activity for less than a second. Creatine phosphate, a high-

In resting muscle, typical concentrations of these metabolites are [ATP] = 4 mM, [ADP] = 0.013 mM, [creatine phosphate] = 25 mM, and [creatine] = 13 mM. Because of its abundance and high phosphoryl-

Phosphates Play a Prominent Role in Biochemical Processes
We have seen in Chapter 13 and in this chapter the prominence of phosphoryl group transfer from ATP to acceptor molecules. How is it that phosphate came to play such a prominent role in biology? Phosphate and its esters have several characteristics that render it useful for biochemical systems. First, phosphate esters have the important property of being thermodynamically unstable while being kinetically stable. Phosphate esters are thus molecules whose energy release can be manipulated by enzymes. Second, the stability of phosphate esters is due to the negative charges that make them resistant to hydrolysis in the absence of enzymes. This accounts for the presence of phosphate in the backbone of DNA. Third, because phosphate esters are so kinetically stable, they make ideal regulatory molecules, added to proteins by kinases and removed only by phosphatases. As we will see many times, phosphates are also frequently added to metabolites that might otherwise diffuse through the cell membrane.
No other ions have the chemical characteristics of phosphate. Citrate is not sufficiently charged to prevent hydrolysis. Arsenate forms esters that are unstable and susceptible to spontaneous hydrolysis. Indeed, arsenate is poisonous to cells because it can replace phosphate in reactions required for ATP synthesis, generating unstable compounds and preventing ATP synthesis. Silicate is more abundant than phosphate, but silicate salts are virtually insoluble, and in fact, are used for biomineralization. Only phosphate has the chemical properties to meet the needs of living systems.